Glass and silk aren't the only materials known to behave like this. Anyone who has ever brushed up against a latex balloon only to find that it tries to stick to them has experienced this same phenomenon. Paraffin wax and wool cloth are another pair of materials early experimenters recognized as manifesting attractive forces after being rubbed together:
This phenomenon became even more interesting when it was discovered that identical materials, after having been rubbed with their respective cloths, always repelled each other:
It was also noted that when a piece of glass rubbed with silk was exposed to a piece of wax rubbed with wool, the two materials would attract one another:
Furthermore, it was found that any material demonstrating properties of attraction or repulsion after being rubbed could be classed into one of two distinct categories: attracted to glass and repelled by wax, or repelled by glass and attracted to wax. It was either one or the other: there were no materials found that would be attracted to or repelled by both glass and wax, or that reacted to one without reacting to the other.
More attention was directed toward the pieces of cloth used to do the rubbing. It was discovered that after rubbing two pieces of glass with two pieces of silk cloth, not only did the glass pieces repel each other, but so did the cloths. The same phenomenon held for the pieces of wool used to rub the wax:
Now, this was really strange to witness. After all, none of these objects were visibly altered by the rubbing, yet they definitely behaved differently than before they were rubbed. Whatever change took place to make these materials attract or repel one another was invisible.
Some experimenters speculated that invisible "fluids" were being transferred from one object to another during the process of rubbing, and that these "fluids" were able to effect a physical force over a distance. Charles Dufay was one the early experimenters who demonstrated that there were definitely two different types of changes wrought by rubbing certain pairs of objects together. The fact that there was more than one type of change manifested in these materials was evident by the fact that there were two types of forces produced: attraction and repulsion. The hypothetical fluid transfer became known as a charge.
One pioneering researcher, Benjamin Franklin, came to the conclusion that there was only one fluid exchanged between rubbed objects, and that the two different "charges" were nothing more than either an excess or a deficiency of that one fluid. After experimenting with wax and wool, Franklin suggested that the coarse wool removed some of this invisible fluid from the smooth wax, causing an excess of fluid on the wool and a deficiency of fluid on the wax. The resulting disparity in fluid content between the wool and wax would then cause an attractive force, as the fluid tried to regain its former balance between the two materials.
Postulating the existence of a single "fluid" that was either gained or lost through rubbing accounted best for the observed behavior: that all these materials fell neatly into one of two categories when rubbed, and most importantly, that the two active materials rubbed against each other always fell into opposing categories as evidenced by their invariable attraction to one another. In other words, there was never a time where two materials rubbed against each other both became either positive or negative.
Following Franklin's speculation of the wool rubbing something off of the wax, the type of charge that was associated with rubbed wax became known as "negative" (because it was supposed to have a deficiency of fluid) while the type of charge associated with the rubbing wool became known as "positive" (because it was supposed to have an excess of fluid). Little did he know that his innocent conjecture would cause much confusion for students of electricity in the future!
Precise measurements of electrical charge were carried out by the French physicist Charles Coulomb in the 1780's using a device called a torsional balance measuring the force generated between two electrically charged objects. The results of Coulomb's work led to the development of a unit of electrical charge named in his honor, the coulomb. If two "point" objects (hypothetical objects having no appreciable surface area) were equally charged to a measure of 1 coulomb, and placed 1 meter (approximately 1 yard) apart, they would generate a force of about 9 billion newtons (approximately 2 billion pounds), either attracting or repelling depending on the types of charges involved.
It was discovered much later that this "fluid" was actually composed of extremely small bits of matter called electrons, so named in honor of the ancient Greek word for amber: another material exhibiting charged properties when rubbed with cloth. Experimentation has since revealed that all objects are composed of extremely small "building-blocks" known as atoms, and that these atoms are in turn composed of smaller components known as particles. The three fundamental particles comprising most atoms are called protons, neutrons and electrons.
Now, this was really strange to witness. After all, none of these objects were visibly altered by the rubbing, yet they definitely behaved differently than before they were rubbed. Whatever change took place to make these materials attract or repel one another was invisible.
Some experimenters speculated that invisible "fluids" were being transferred from one object to another during the process of rubbing, and that these "fluids" were able to effect a physical force over a distance. Charles Dufay was one the early experimenters who demonstrated that there were definitely two different types of changes wrought by rubbing certain pairs of objects together. The fact that there was more than one type of change manifested in these materials was evident by the fact that there were two types of forces produced: attraction and repulsion. The hypothetical fluid transfer became known as a charge.
One pioneering researcher, Benjamin Franklin, came to the conclusion that there was only one fluid exchanged between rubbed objects, and that the two different "charges" were nothing more than either an excess or a deficiency of that one fluid. After experimenting with wax and wool, Franklin suggested that the coarse wool removed some of this invisible fluid from the smooth wax, causing an excess of fluid on the wool and a deficiency of fluid on the wax. The resulting disparity in fluid content between the wool and wax would then cause an attractive force, as the fluid tried to regain its former balance between the two materials.
Postulating the existence of a single "fluid" that was either gained or lost through rubbing accounted best for the observed behavior: that all these materials fell neatly into one of two categories when rubbed, and most importantly, that the two active materials rubbed against each other always fell into opposing categories as evidenced by their invariable attraction to one another. In other words, there was never a time where two materials rubbed against each other both became either positive or negative.
Following Franklin's speculation of the wool rubbing something off of the wax, the type of charge that was associated with rubbed wax became known as "negative" (because it was supposed to have a deficiency of fluid) while the type of charge associated with the rubbing wool became known as "positive" (because it was supposed to have an excess of fluid). Little did he know that his innocent conjecture would cause much confusion for students of electricity in the future!
Precise measurements of electrical charge were carried out by the French physicist Charles Coulomb in the 1780's using a device called a torsional balance measuring the force generated between two electrically charged objects. The results of Coulomb's work led to the development of a unit of electrical charge named in his honor, the coulomb. If two "point" objects (hypothetical objects having no appreciable surface area) were equally charged to a measure of 1 coulomb, and placed 1 meter (approximately 1 yard) apart, they would generate a force of about 9 billion newtons (approximately 2 billion pounds), either attracting or repelling depending on the types of charges involved.
It was discovered much later that this "fluid" was actually composed of extremely small bits of matter called electrons, so named in honor of the ancient Greek word for amber: another material exhibiting charged properties when rubbed with cloth. Experimentation has since revealed that all objects are composed of extremely small "building-blocks" known as atoms, and that these atoms are in turn composed of smaller components known as particles. The three fundamental particles comprising most atoms are called protons, neutrons and electrons.
Now, this was really strange to witness. After all, none of these objects were visibly altered by the rubbing, yet they definitely behaved differently than before they were rubbed. Whatever change took place to make these materials attract or repel one another was invisible.
Some experimenters speculated that invisible "fluids" were being transferred from one object to another during the process of rubbing, and that these "fluids" were able to effect a physical force over a distance. Charles Dufay was one the early experimenters who demonstrated that there were definitely two different types of changes wrought by rubbing certain pairs of objects together. The fact that there was more than one type of change manifested in these materials was evident by the fact that there were two types of forces produced: attraction and repulsion. The hypothetical fluid transfer became known as a charge.
One pioneering researcher, Benjamin Franklin, came to the conclusion that there was only one fluid exchanged between rubbed objects, and that the two different "charges" were nothing more than either an excess or a deficiency of that one fluid. After experimenting with wax and wool, Franklin suggested that the coarse wool removed some of this invisible fluid from the smooth wax, causing an excess of fluid on the wool and a deficiency of fluid on the wax. The resulting disparity in fluid content between the wool and wax would then cause an attractive force, as the fluid tried to regain its former balance between the two materials.
Postulating the existence of a single "fluid" that was either gained or lost through rubbing accounted best for the observed behavior: that all these materials fell neatly into one of two categories when rubbed, and most importantly, that the two active materials rubbed against each other always fell into opposing categories as evidenced by their invariable attraction to one another. In other words, there was never a time where two materials rubbed against each other both became either positive or negative.
Following Franklin's speculation of the wool rubbing something off of the wax, the type of charge that was associated with rubbed wax became known as "negative" (because it was supposed to have a deficiency of fluid) while the type of charge associated with the rubbing wool became known as "positive" (because it was supposed to have an excess of fluid). Little did he know that his innocent conjecture would cause much confusion for students of electricity in the future!
Precise measurements of electrical charge were carried out by the French physicist Charles Coulomb in the 1780's using a device called a torsional balance measuring the force generated between two electrically charged objects. The results of Coulomb's work led to the development of a unit of electrical charge named in his honor, the coulomb. If two "point" objects (hypothetical objects having no appreciable surface area) were equally charged to a measure of 1 coulomb, and placed 1 meter (approximately 1 yard) apart, they would generate a force of about 9 billion newtons (approximately 2 billion pounds), either attracting or repelling depending on the types of charges involved.
It was discovered much later that this "fluid" was actually composed of extremely small bits of matter called electrons, so named in honor of the ancient Greek word for amber: another material exhibiting charged properties when rubbed with cloth. Experimentation has since revealed that all objects are composed of extremely small "building-blocks" known as atoms, and that these atoms are in turn composed of smaller components known as particles. The three fundamental particles comprising most atoms are called protons, neutrons and electrons.
skip to main |
skip to sidebar
![]()

SAVE WATER.



Gone are the dull book days !!!!!!

symmetry in a tiger

smart classroom

Kulachi Hansraj Model School


Use dustbins!

The key to the protection and care of the Earth


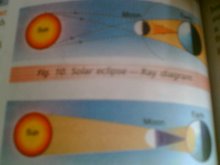

Opaque objects cast distinct shadows


Karanjot V B

Shadows and eclipses



Love and care your environment!

A PLEDGE BY YOUNG KULACHIANS TO SAVE THE EARTH!


Monocot and Dicot seed

The only living things to capture Sun's energy
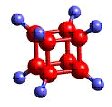
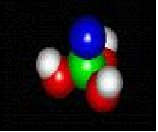
atoms

WELCOME TO MY FUN SCIENCE BLOG
I am Savita Dhutti,a science teacher at a well established public school in India.I welcome all of you to My science mania ! Its a website,I created for sharing e-learning experience with my students.Science is in fact, a real fun.It is a subject never--never confined within the four walls of the clssroom.Science is a continuous process of learning! I wish to create a pleasing learning environment for my students.So, Come and ENJOY Science with me.
class 8 BIOLOGY H HW
Biology
Make a project on the following topic
Microorganisms
The following aspects should be properly covered.
- Different group of microorganisms 5 characteristics of each
Algae, Fungi, Bacteria, Protozoa, Virus Microorganisms as friends in Food, Medicine, Agriculture, Nature, Industry and Microorganisms as Foes.
Use A4 size sheets and put them inside an Eco friendly Folder.
Be thorough with whatever you write, you will be orally assessed.
Labels
- A CALL for the students....... (6)
- A student showing solar eclipse (1)
- An Intelligent mind rests in a healthy body. (1)
- BIOLOGY (1)
- Body systems diagrams for practice (2)
- Chant Gayatri Mantra daily and live life fully. (1)
- class 8 HHW BIOLOGY (10)
- CLASS VII HOLIDAYS HOME WORK (8)
- click to enlarge and print (1)
- cow dung.holidays home work reference (18)
- electric current (13)
- fabric from fiber (4)
- food class 6th (24)
- global concern (8)
- global warming (6)
- Information (1)
- Information: symmetry (2)
- Jupiter facts (1)
- life cycles of animals (1)
- light and shadows (4)
- link to my slideshow (1)
- magnets (2)
- nature of matter (2)
- plant structure and function (7)
- plants (9)
- plants image gallary (2)
- properties of matter (3)
- puzzle clues (1)
- Seed germination (1)
- SHIVLING AT the AMARNATH TEMPLE HAS MELTED. (1)
- Student Activity (43)
- student Corner (47)
- student information (10)
- Syllabus Class V / SCIENCE / FIRST TERM (1)
- Take A break (1)
- water facts (6)
- word bank plants (2)
- work and energy (11)
- धरती को बचाने की कोशिश (1)
- प्लांट growth (1)
Total Pageviews
shadow dancing
Facebook Badge
e teachers of india my badge
welcome to my science mania!!!!!!!
I am Savita Dhutti,a science teacher at a well established public school in India.I welcome all of you to My science mania ! Its a website,I created for sharing e-learning experience with my students.Science is in fact, a real fun.It is a subject never--never confined within the four walls of the clssroom.Science is a continuous process of learning! I wish to create a pleasing learning environment for my students.
So, Come and ENJOY Science with me.
So, Come and ENJOY Science with me.
global warming
NeoCounter
NeoPod
NeoFlags
EVERY DROP OF WATER IS PRECIOUS.

SAVE WATER.
water
VI H celebrating Talent Show 2007

Labels
- A CALL for the students....... (6)
- A student showing solar eclipse (1)
- An Intelligent mind rests in a healthy body. (1)
- BIOLOGY (1)
- Body systems diagrams for practice (2)
- Chant Gayatri Mantra daily and live life fully. (1)
- class 8 HHW BIOLOGY (10)
- CLASS VII HOLIDAYS HOME WORK (8)
- click to enlarge and print (1)
- cow dung.holidays home work reference (18)
- electric current (13)
- fabric from fiber (4)
- food class 6th (24)
- global concern (8)
- global warming (6)
- Information (1)
- Information: symmetry (2)
- Jupiter facts (1)
- life cycles of animals (1)
- light and shadows (4)
- link to my slideshow (1)
- magnets (2)
- nature of matter (2)
- plant structure and function (7)
- plants (9)
- plants image gallary (2)
- properties of matter (3)
- puzzle clues (1)
- Seed germination (1)
- SHIVLING AT the AMARNATH TEMPLE HAS MELTED. (1)
- Student Activity (43)
- student Corner (47)
- student information (10)
- Syllabus Class V / SCIENCE / FIRST TERM (1)
- Take A break (1)
- water facts (6)
- word bank plants (2)
- work and energy (11)
- धरती को बचाने की कोशिश (1)
- प्लांट growth (1)

Its e-teaching time now!!!!!!!!!!!

Gone are the dull book days !!!!!!
CII Shiksha India's website
CII SHIKSHA INDIA IS A NON PROFIT ORGANIZATION ........
CII SHIKSHA INDIA IS A NON PROFIT ORGANIZATION ........WHICH STARTED ITS .........
e-teaching learning portal in January 2007 inagurated by President Abdul Kalam Azad and is continuously guiding teachers to work in collaboration on Shiksha portal for taking India to the new horizons of E-teaching learning.I have been assosiated with it and working on e-teaching learning.The portal had announced a contest for its teachers for contribution to the portal and myself ,the recipient of the award is happy to be assosiated to the portal and in being able to explore net and other e-tools for teaching.
e-teaching learning portal in January 2007 inagurated by President Abdul Kalam Azad and is continuously guiding teachers to work in collaboration on Shiksha portal for taking India to the new horizons of E-teaching learning.I have been assosiated with it and working on e-teaching learning.The portal had announced a contest for its teachers for contribution to the portal and myself ,the recipient of the award is happy to be assosiated to the portal and in being able to explore net and other e-tools for teaching.
symmetry

symmetry in a tiger
e-learning is fun!

smart classroom
alphabets in nature
This album is powered by BubbleShare - Add to my blog
MY SCHOOL

Kulachi Hansraj Model School
KULACHI BLOGGERS CLUB

Manage the waste

Use dustbins!
Student Awareness

The key to the protection and care of the Earth
Protecting Earth together

Student corner
- 1. Poem by Megha Panjwani VI H
- 2. Vasu Mittal says........
- 3. Poem by Manmeet Kaur VI H

Do you think talent shows in the school are worth it or a wastage of time?
Items for the talent show of my class
- On the spot Poetry competition
- science facts
- Making invitation cards for the guests
- Rangoli making
- wecome song by girls
- art and craft display
- Singing bhajan
- folk dance boys(solo)
- folk dance girls(solo)
- group dance by boys
- group dance by girls
- drama
- playing music instument
- short humourous skit
solar eclipse

shadow formation activity PARAS V B

Opaque objects cast distinct shadows
global warming

Shadow is not formed by transparent objects

Karanjot V B
class V activity

Shadows and eclipses

url=http://savitadhuttimyscienceatkulachi.blogspot.com" id="clustrMapsLink">
click for the details
time is ticking away fast
Labels
- A CALL for the students....... (6)
- A student showing solar eclipse (1)
- An Intelligent mind rests in a healthy body. (1)
- BIOLOGY (1)
- Body systems diagrams for practice (2)
- Chant Gayatri Mantra daily and live life fully. (1)
- class 8 HHW BIOLOGY (10)
- CLASS VII HOLIDAYS HOME WORK (8)
- click to enlarge and print (1)
- cow dung.holidays home work reference (18)
- electric current (13)
- fabric from fiber (4)
- food class 6th (24)
- global concern (8)
- global warming (6)
- Information (1)
- Information: symmetry (2)
- Jupiter facts (1)
- life cycles of animals (1)
- light and shadows (4)
- link to my slideshow (1)
- magnets (2)
- nature of matter (2)
- plant structure and function (7)
- plants (9)
- plants image gallary (2)
- properties of matter (3)
- puzzle clues (1)
- Seed germination (1)
- SHIVLING AT the AMARNATH TEMPLE HAS MELTED. (1)
- Student Activity (43)
- student Corner (47)
- student information (10)
- Syllabus Class V / SCIENCE / FIRST TERM (1)
- Take A break (1)
- water facts (6)
- word bank plants (2)
- work and energy (11)
- धरती को बचाने की कोशिश (1)
- प्लांट growth (1)
my school's location through google earth

calender
clicks on my blog
Science is not just a subject of teaching learning.It is a way of knowing about,thinking about and caring about the environment of which we are a part.
Labels
- A CALL for the students....... (6)
- A student showing solar eclipse (1)
- An Intelligent mind rests in a healthy body. (1)
- BIOLOGY (1)
- Body systems diagrams for practice (2)
- Chant Gayatri Mantra daily and live life fully. (1)
- class 8 HHW BIOLOGY (10)
- CLASS VII HOLIDAYS HOME WORK (8)
- click to enlarge and print (1)
- cow dung.holidays home work reference (18)
- electric current (13)
- fabric from fiber (4)
- food class 6th (24)
- global concern (8)
- global warming (6)
- Information (1)
- Information: symmetry (2)
- Jupiter facts (1)
- life cycles of animals (1)
- light and shadows (4)
- link to my slideshow (1)
- magnets (2)
- nature of matter (2)
- plant structure and function (7)
- plants (9)
- plants image gallary (2)
- properties of matter (3)
- puzzle clues (1)
- Seed germination (1)
- SHIVLING AT the AMARNATH TEMPLE HAS MELTED. (1)
- Student Activity (43)
- student Corner (47)
- student information (10)
- Syllabus Class V / SCIENCE / FIRST TERM (1)
- Take A break (1)
- water facts (6)
- word bank plants (2)
- work and energy (11)
- धरती को बचाने की कोशिश (1)
- प्लांट growth (1)
Class VI Students ! Refer this site for help in your Science Project given as Holidays H.W.
Saving Earth's Resources
seed germination
Planting a green friend -- a sapling !

Love and care your environment!
a pledge by the Students in the VANAMAHOTSAVA function

A PLEDGE BY YOUNG KULACHIANS TO SAVE THE EARTH!
plant growth

Seed Germination

Monocot and Dicot seed
A Typical Flowering Plant

The only living things to capture Sun's energy
Labels
- A CALL for the students....... (6)
- A student showing solar eclipse (1)
- An Intelligent mind rests in a healthy body. (1)
- BIOLOGY (1)
- Body systems diagrams for practice (2)
- Chant Gayatri Mantra daily and live life fully. (1)
- class 8 HHW BIOLOGY (10)
- CLASS VII HOLIDAYS HOME WORK (8)
- click to enlarge and print (1)
- cow dung.holidays home work reference (18)
- electric current (13)
- fabric from fiber (4)
- food class 6th (24)
- global concern (8)
- global warming (6)
- Information (1)
- Information: symmetry (2)
- Jupiter facts (1)
- life cycles of animals (1)
- light and shadows (4)
- link to my slideshow (1)
- magnets (2)
- nature of matter (2)
- plant structure and function (7)
- plants (9)
- plants image gallary (2)
- properties of matter (3)
- puzzle clues (1)
- Seed germination (1)
- SHIVLING AT the AMARNATH TEMPLE HAS MELTED. (1)
- Student Activity (43)
- student Corner (47)
- student information (10)
- Syllabus Class V / SCIENCE / FIRST TERM (1)
- Take A break (1)
- water facts (6)
- word bank plants (2)
- work and energy (11)
- धरती को बचाने की कोशिश (1)
- प्लांट growth (1)
molecules in nature

"Welcome to the planet science"
Welcome to the exciting world of Science!
This is my planet science-----A fun filled planet full of multiple activities,interesting videos, thought provoking articles on contemporary science issues,puzzles , compiled images and much more.....
Atoms---- the constituent particles of matter

atoms
Blog Archive
-
►
2007
(134)
- ► 05/06 - 05/13 (13)
- ► 05/13 - 05/20 (19)
- ► 05/20 - 05/27 (13)
- ► 05/27 - 06/03 (11)
- ► 06/10 - 06/17 (1)
- ► 07/01 - 07/08 (4)
- ► 07/08 - 07/15 (11)
- ► 07/15 - 07/22 (11)
- ► 07/29 - 08/05 (11)
- ► 08/05 - 08/12 (3)
- ► 08/12 - 08/19 (12)
- ► 08/19 - 08/26 (3)
- ► 09/09 - 09/16 (10)
- ► 09/16 - 09/23 (3)
- ► 09/30 - 10/07 (3)
- ► 10/07 - 10/14 (2)
- ► 12/09 - 12/16 (4)
-
▼
2008
(137)
- ► 01/06 - 01/13 (2)
- ► 01/13 - 01/20 (3)
- ► 01/20 - 01/27 (6)
- ► 01/27 - 02/03 (11)
- ► 02/10 - 02/17 (2)
- ► 02/17 - 02/24 (2)
- ► 03/16 - 03/23 (2)
- ► 03/23 - 03/30 (3)
- ► 03/30 - 04/06 (1)
- ► 04/06 - 04/13 (33)
- ► 05/04 - 05/11 (4)
- ► 05/11 - 05/18 (1)
- ► 05/18 - 05/25 (3)
- ► 06/08 - 06/15 (6)
- ► 06/29 - 07/06 (1)
- ► 07/13 - 07/20 (1)
- ► 08/10 - 08/17 (7)
- ► 08/17 - 08/24 (5)
- ► 08/31 - 09/07 (1)
- ► 09/14 - 09/21 (1)
- ► 09/21 - 09/28 (2)
- ► 09/28 - 10/05 (6)
-
▼
11/23 - 11/30
(17)
- battery cell
- how batteries work
- No title
- Do you wish to know how electric torch works?
- Structure of an electric torch
- Student explaining working of the torch in the class
- student explaining parts of electric torch
- Electric circuit
- No title
- Electric circuits
- No title
- Static electricity
- No title
- No title
- Static electricity
- No title
- electricity
- ► 11/30 - 12/07 (4)
- ► 12/14 - 12/21 (10)
- ► 12/21 - 12/28 (3)
-
►
2009
(44)
- ► 01/18 - 01/25 (2)
- ► 02/08 - 02/15 (5)
- ► 03/22 - 03/29 (2)
- ► 03/29 - 04/05 (1)
- ► 04/12 - 04/19 (1)
- ► 05/03 - 05/10 (8)
- ► 05/10 - 05/17 (1)
- ► 05/24 - 05/31 (17)
- ► 07/26 - 08/02 (1)
- ► 10/25 - 11/01 (3)
- ► 12/20 - 12/27 (3)
-
►
2010
(16)
- ► 01/17 - 01/24 (1)
- ► 01/24 - 01/31 (4)
- ► 01/31 - 02/07 (3)
- ► 05/30 - 06/06 (6)
- ► 08/29 - 09/05 (1)
- ► 10/03 - 10/10 (1)
-
►
2011
(21)
- ► 05/01 - 05/08 (11)
- ► 05/15 - 05/22 (10)
Hi ! I AM A SCIENCE TEACHER.

- savitadhutti
- Delhi, Delhi, India
- I AM A SCIENCE TEACHER IN A WELL REPUTED SCHOOL IN INDIA. I AM A MEMBER OF e-shiksha CLUB. I HAVE JOINED TEACHERTUBE FORUM AS WELL. I AM EXPLORING NET TO LEARN AND SHARE.
Nature and you
MOLECULES IN NATURE FORM BONDS JUST LIKE YOU ARE BONDED TO YOUR FRIENDS.


















