The three fundamental particles comprising most atoms are called protons, neutrons and electrons. Whilst the majority of atoms have a combination of protons, neutrons, and electrons, not all atoms have neutrons; an example is the protium isotope (1H1) of hydrogen (Hydrogen-1) which is the lightest and most common form of hydrogen which only has one proton and one electron. Atoms are far too small to be seen, but if we could look at one, it might appear something like this:
Even though each atom in a piece of material tends to hold together as a unit, there's actually a lot of empty space between the electrons and the cluster of protons and neutrons residing in the middle.
This crude model is that of the element carbon, with six protons, six neutrons, and six electrons. In any atom, the protons and neutrons are very tightly bound together, which is an important quality. The tightly-bound clump of protons and neutrons in the center of the atom is called the nucleus, and the number of protons in an atom's nucleus determines its elemental identity: change the number of protons in an atom's nucleus, and you change the type of atom that it is. In fact, if you could remove three protons from the nucleus of an atom of lead, you will have achieved the old alchemists' dream of producing an atom of gold! The tight binding of protons in the nucleus is responsible for the stable identity of chemical elements, and the failure of alchemists to achieve their dream.
Neutrons are much less influential on the chemical character and identity of an atom than protons, although they are just as hard to add to or remove from the nucleus, being so tightly bound. If neutrons are added or gained, the atom will still retain the same chemical identity, but its mass will change slightly and it may acquire strange nuclear properties such as radioactivity.
However, electrons have significantly more freedom to move around in an atom than either protons or neutrons. In fact, they can be knocked out of their respective positions (even leaving the atom entirely!) by far less energy than what it takes to dislodge particles in the nucleus. If this happens, the atom still retains its chemical identity, but an important imbalance occurs. Electrons and protons are unique in the fact that they are attracted to one another over a distance. It is this attraction over distance which causes the attraction between rubbed objects, where electrons are moved away from their original atoms to reside around atoms of another object.
Electrons tend to repel other electrons over a distance, as do protons with other protons. The only reason protons bind together in the nucleus of an atom is because of a much stronger force called the strong nuclear force which has effect only under very short distances. Because of this attraction/repulsion behavior between individual particles, electrons and protons are said to have opposite electric charges. That is, each electron has a negative charge, and each proton a positive charge. In equal numbers within an atom, they counteract each other's presence so that the net charge within the atom is zero. This is why the picture of a carbon atom had six electrons: to balance out the electric charge of the six protons in the nucleus. If electrons leave or extra electrons arrive, the atom's net electric charge will be imbalanced, leaving the atom "charged" as a whole, causing it to interact with charged particles and other charged atoms nearby. Neutrons are neither attracted to or repelled by electrons, protons, or even other neutrons, and are consequently categorized as having no charge at all.
The process of electrons arriving or leaving is exactly what happens when certain combinations of materials are rubbed together: electrons from the atoms of one material are forced by the rubbing to leave their respective atoms and transfer over to the atoms of the other material. In other words, electrons comprise the "fluid" hypothesized by Benjamin Franklin. The operational definition of a coulomb as the unit of electrical charge (in terms of force generated between point charges) was found to be equal to an excess or deficiency of about 6,250,000,000,000,000,000 electrons. Or, stated in reverse terms, one electron has a charge of about 0.00000000000000000016 coulombs. Being that one electron is the smallest known carrier of electric charge, this last figure of charge for the electron is defined as the elementary charge.
The result of an imbalance of this "fluid" (electrons) between objects is called static electricity. It is called "static" because the displaced electrons tend to remain stationary after being moved from one insulating material to another. In the case of wax and wool, it was determined through further experimentation that electrons in the wool actually transferred to the atoms in the wax, which is exactly opposite of Franklin's conjecture! In honor of Franklin's designation of the wax's charge being "negative" and the wool's charge being "positive," electrons are said to have a "negative" charging influence. Thus, an object whose atoms have received a surplus of electrons is said to be negatively charged, while an object whose atoms are lacking electrons is said to be positively charged, as confusing as these designations may seem. By the time the true nature of electric "fluid" was discovered, Franklin's nomenclature of electric charge was too well established to be easily changed, and so it remains to this day.
Michael Faraday proved (1832) that static electricity was the same as that produced by a battery or a generator. Static electricity is, for the most part, a nusiance. Black powder and smokeless powder have graphite added to prevent ignition due to static electricity. It causes damage to sensitive semiconductor circuitry. While is is possible to produce motors powered by high voltage and low current characteristic of static electricity, this is not economic. The few practical applications of static electricity include xerographic printing, the electrostatic air filter, and the high voltage Van de Graaff generator.
skip to main |
skip to sidebar
![]()

SAVE WATER.



Gone are the dull book days !!!!!!

symmetry in a tiger

smart classroom

Kulachi Hansraj Model School


Use dustbins!

The key to the protection and care of the Earth


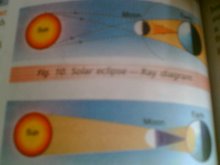

Opaque objects cast distinct shadows


Karanjot V B

Shadows and eclipses



Love and care your environment!

A PLEDGE BY YOUNG KULACHIANS TO SAVE THE EARTH!


Monocot and Dicot seed

The only living things to capture Sun's energy
atoms

WELCOME TO MY FUN SCIENCE BLOG
I am Savita Dhutti,a science teacher at a well established public school in India.I welcome all of you to My science mania ! Its a website,I created for sharing e-learning experience with my students.Science is in fact, a real fun.It is a subject never--never confined within the four walls of the clssroom.Science is a continuous process of learning! I wish to create a pleasing learning environment for my students.So, Come and ENJOY Science with me.
class 8 BIOLOGY H HW
Biology
Make a project on the following topic
Microorganisms
The following aspects should be properly covered.
- Different group of microorganisms 5 characteristics of each
Algae, Fungi, Bacteria, Protozoa, Virus Microorganisms as friends in Food, Medicine, Agriculture, Nature, Industry and Microorganisms as Foes.
Use A4 size sheets and put them inside an Eco friendly Folder.
Be thorough with whatever you write, you will be orally assessed.
Labels
- A CALL for the students....... (6)
- A student showing solar eclipse (1)
- An Intelligent mind rests in a healthy body. (1)
- BIOLOGY (1)
- Body systems diagrams for practice (2)
- Chant Gayatri Mantra daily and live life fully. (1)
- class 8 HHW BIOLOGY (10)
- CLASS VII HOLIDAYS HOME WORK (8)
- click to enlarge and print (1)
- cow dung.holidays home work reference (18)
- electric current (13)
- fabric from fiber (4)
- food class 6th (24)
- global concern (8)
- global warming (6)
- Information (1)
- Information: symmetry (2)
- Jupiter facts (1)
- life cycles of animals (1)
- light and shadows (4)
- link to my slideshow (1)
- magnets (2)
- nature of matter (2)
- plant structure and function (7)
- plants (9)
- plants image gallary (2)
- properties of matter (3)
- puzzle clues (1)
- Seed germination (1)
- SHIVLING AT the AMARNATH TEMPLE HAS MELTED. (1)
- Student Activity (43)
- student Corner (47)
- student information (10)
- Syllabus Class V / SCIENCE / FIRST TERM (1)
- Take A break (1)
- water facts (6)
- word bank plants (2)
- work and energy (11)
- धरती को बचाने की कोशिश (1)
- प्लांट growth (1)
Total Pageviews
shadow dancing
Facebook Badge
e teachers of india my badge
welcome to my science mania!!!!!!!
I am Savita Dhutti,a science teacher at a well established public school in India.I welcome all of you to My science mania ! Its a website,I created for sharing e-learning experience with my students.Science is in fact, a real fun.It is a subject never--never confined within the four walls of the clssroom.Science is a continuous process of learning! I wish to create a pleasing learning environment for my students.
So, Come and ENJOY Science with me.
So, Come and ENJOY Science with me.
global warming
NeoCounter
NeoPod
NeoFlags
EVERY DROP OF WATER IS PRECIOUS.

SAVE WATER.
water
VI H celebrating Talent Show 2007

Labels
- A CALL for the students....... (6)
- A student showing solar eclipse (1)
- An Intelligent mind rests in a healthy body. (1)
- BIOLOGY (1)
- Body systems diagrams for practice (2)
- Chant Gayatri Mantra daily and live life fully. (1)
- class 8 HHW BIOLOGY (10)
- CLASS VII HOLIDAYS HOME WORK (8)
- click to enlarge and print (1)
- cow dung.holidays home work reference (18)
- electric current (13)
- fabric from fiber (4)
- food class 6th (24)
- global concern (8)
- global warming (6)
- Information (1)
- Information: symmetry (2)
- Jupiter facts (1)
- life cycles of animals (1)
- light and shadows (4)
- link to my slideshow (1)
- magnets (2)
- nature of matter (2)
- plant structure and function (7)
- plants (9)
- plants image gallary (2)
- properties of matter (3)
- puzzle clues (1)
- Seed germination (1)
- SHIVLING AT the AMARNATH TEMPLE HAS MELTED. (1)
- Student Activity (43)
- student Corner (47)
- student information (10)
- Syllabus Class V / SCIENCE / FIRST TERM (1)
- Take A break (1)
- water facts (6)
- word bank plants (2)
- work and energy (11)
- धरती को बचाने की कोशिश (1)
- प्लांट growth (1)

Its e-teaching time now!!!!!!!!!!!

Gone are the dull book days !!!!!!
CII Shiksha India's website
CII SHIKSHA INDIA IS A NON PROFIT ORGANIZATION ........
CII SHIKSHA INDIA IS A NON PROFIT ORGANIZATION ........WHICH STARTED ITS .........
e-teaching learning portal in January 2007 inagurated by President Abdul Kalam Azad and is continuously guiding teachers to work in collaboration on Shiksha portal for taking India to the new horizons of E-teaching learning.I have been assosiated with it and working on e-teaching learning.The portal had announced a contest for its teachers for contribution to the portal and myself ,the recipient of the award is happy to be assosiated to the portal and in being able to explore net and other e-tools for teaching.
e-teaching learning portal in January 2007 inagurated by President Abdul Kalam Azad and is continuously guiding teachers to work in collaboration on Shiksha portal for taking India to the new horizons of E-teaching learning.I have been assosiated with it and working on e-teaching learning.The portal had announced a contest for its teachers for contribution to the portal and myself ,the recipient of the award is happy to be assosiated to the portal and in being able to explore net and other e-tools for teaching.
symmetry

symmetry in a tiger
e-learning is fun!

smart classroom
alphabets in nature
This album is powered by BubbleShare - Add to my blog
MY SCHOOL

Kulachi Hansraj Model School
KULACHI BLOGGERS CLUB

Manage the waste

Use dustbins!
Student Awareness

The key to the protection and care of the Earth
Protecting Earth together

Student corner
- 1. Poem by Megha Panjwani VI H
- 2. Vasu Mittal says........
- 3. Poem by Manmeet Kaur VI H

Do you think talent shows in the school are worth it or a wastage of time?
Items for the talent show of my class
- On the spot Poetry competition
- science facts
- Making invitation cards for the guests
- Rangoli making
- wecome song by girls
- art and craft display
- Singing bhajan
- folk dance boys(solo)
- folk dance girls(solo)
- group dance by boys
- group dance by girls
- drama
- playing music instument
- short humourous skit
solar eclipse

shadow formation activity PARAS V B

Opaque objects cast distinct shadows
global warming

Shadow is not formed by transparent objects

Karanjot V B
class V activity

Shadows and eclipses

url=http://savitadhuttimyscienceatkulachi.blogspot.com" id="clustrMapsLink">
click for the details
time is ticking away fast
Labels
- A CALL for the students....... (6)
- A student showing solar eclipse (1)
- An Intelligent mind rests in a healthy body. (1)
- BIOLOGY (1)
- Body systems diagrams for practice (2)
- Chant Gayatri Mantra daily and live life fully. (1)
- class 8 HHW BIOLOGY (10)
- CLASS VII HOLIDAYS HOME WORK (8)
- click to enlarge and print (1)
- cow dung.holidays home work reference (18)
- electric current (13)
- fabric from fiber (4)
- food class 6th (24)
- global concern (8)
- global warming (6)
- Information (1)
- Information: symmetry (2)
- Jupiter facts (1)
- life cycles of animals (1)
- light and shadows (4)
- link to my slideshow (1)
- magnets (2)
- nature of matter (2)
- plant structure and function (7)
- plants (9)
- plants image gallary (2)
- properties of matter (3)
- puzzle clues (1)
- Seed germination (1)
- SHIVLING AT the AMARNATH TEMPLE HAS MELTED. (1)
- Student Activity (43)
- student Corner (47)
- student information (10)
- Syllabus Class V / SCIENCE / FIRST TERM (1)
- Take A break (1)
- water facts (6)
- word bank plants (2)
- work and energy (11)
- धरती को बचाने की कोशिश (1)
- प्लांट growth (1)
my school's location through google earth

calender
clicks on my blog
Science is not just a subject of teaching learning.It is a way of knowing about,thinking about and caring about the environment of which we are a part.
Labels
- A CALL for the students....... (6)
- A student showing solar eclipse (1)
- An Intelligent mind rests in a healthy body. (1)
- BIOLOGY (1)
- Body systems diagrams for practice (2)
- Chant Gayatri Mantra daily and live life fully. (1)
- class 8 HHW BIOLOGY (10)
- CLASS VII HOLIDAYS HOME WORK (8)
- click to enlarge and print (1)
- cow dung.holidays home work reference (18)
- electric current (13)
- fabric from fiber (4)
- food class 6th (24)
- global concern (8)
- global warming (6)
- Information (1)
- Information: symmetry (2)
- Jupiter facts (1)
- life cycles of animals (1)
- light and shadows (4)
- link to my slideshow (1)
- magnets (2)
- nature of matter (2)
- plant structure and function (7)
- plants (9)
- plants image gallary (2)
- properties of matter (3)
- puzzle clues (1)
- Seed germination (1)
- SHIVLING AT the AMARNATH TEMPLE HAS MELTED. (1)
- Student Activity (43)
- student Corner (47)
- student information (10)
- Syllabus Class V / SCIENCE / FIRST TERM (1)
- Take A break (1)
- water facts (6)
- word bank plants (2)
- work and energy (11)
- धरती को बचाने की कोशिश (1)
- प्लांट growth (1)
Class VI Students ! Refer this site for help in your Science Project given as Holidays H.W.
Saving Earth's Resources
seed germination
Planting a green friend -- a sapling !

Love and care your environment!
a pledge by the Students in the VANAMAHOTSAVA function

A PLEDGE BY YOUNG KULACHIANS TO SAVE THE EARTH!
plant growth

Seed Germination

Monocot and Dicot seed
A Typical Flowering Plant

The only living things to capture Sun's energy
Labels
- A CALL for the students....... (6)
- A student showing solar eclipse (1)
- An Intelligent mind rests in a healthy body. (1)
- BIOLOGY (1)
- Body systems diagrams for practice (2)
- Chant Gayatri Mantra daily and live life fully. (1)
- class 8 HHW BIOLOGY (10)
- CLASS VII HOLIDAYS HOME WORK (8)
- click to enlarge and print (1)
- cow dung.holidays home work reference (18)
- electric current (13)
- fabric from fiber (4)
- food class 6th (24)
- global concern (8)
- global warming (6)
- Information (1)
- Information: symmetry (2)
- Jupiter facts (1)
- life cycles of animals (1)
- light and shadows (4)
- link to my slideshow (1)
- magnets (2)
- nature of matter (2)
- plant structure and function (7)
- plants (9)
- plants image gallary (2)
- properties of matter (3)
- puzzle clues (1)
- Seed germination (1)
- SHIVLING AT the AMARNATH TEMPLE HAS MELTED. (1)
- Student Activity (43)
- student Corner (47)
- student information (10)
- Syllabus Class V / SCIENCE / FIRST TERM (1)
- Take A break (1)
- water facts (6)
- word bank plants (2)
- work and energy (11)
- धरती को बचाने की कोशिश (1)
- प्लांट growth (1)
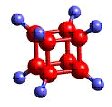
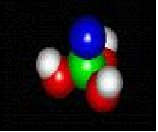
molecules in nature

"Welcome to the planet science"
Welcome to the exciting world of Science!
This is my planet science-----A fun filled planet full of multiple activities,interesting videos, thought provoking articles on contemporary science issues,puzzles , compiled images and much more.....
Atoms---- the constituent particles of matter

atoms
Blog Archive
-
►
2007
(134)
- ► 05/06 - 05/13 (13)
- ► 05/13 - 05/20 (19)
- ► 05/20 - 05/27 (13)
- ► 05/27 - 06/03 (11)
- ► 06/10 - 06/17 (1)
- ► 07/01 - 07/08 (4)
- ► 07/08 - 07/15 (11)
- ► 07/15 - 07/22 (11)
- ► 07/29 - 08/05 (11)
- ► 08/05 - 08/12 (3)
- ► 08/12 - 08/19 (12)
- ► 08/19 - 08/26 (3)
- ► 09/09 - 09/16 (10)
- ► 09/16 - 09/23 (3)
- ► 09/30 - 10/07 (3)
- ► 10/07 - 10/14 (2)
- ► 12/09 - 12/16 (4)
-
▼
2008
(137)
- ► 01/06 - 01/13 (2)
- ► 01/13 - 01/20 (3)
- ► 01/20 - 01/27 (6)
- ► 01/27 - 02/03 (11)
- ► 02/10 - 02/17 (2)
- ► 02/17 - 02/24 (2)
- ► 03/16 - 03/23 (2)
- ► 03/23 - 03/30 (3)
- ► 03/30 - 04/06 (1)
- ► 04/06 - 04/13 (33)
- ► 05/04 - 05/11 (4)
- ► 05/11 - 05/18 (1)
- ► 05/18 - 05/25 (3)
- ► 06/08 - 06/15 (6)
- ► 06/29 - 07/06 (1)
- ► 07/13 - 07/20 (1)
- ► 08/10 - 08/17 (7)
- ► 08/17 - 08/24 (5)
- ► 08/31 - 09/07 (1)
- ► 09/14 - 09/21 (1)
- ► 09/21 - 09/28 (2)
- ► 09/28 - 10/05 (6)
-
▼
11/23 - 11/30
(17)
- battery cell
- how batteries work
- No title
- Do you wish to know how electric torch works?
- Structure of an electric torch
- Student explaining working of the torch in the class
- student explaining parts of electric torch
- Electric circuit
- No title
- Electric circuits
- No title
- Static electricity
- No title
- No title
- Static electricity
- No title
- electricity
- ► 11/30 - 12/07 (4)
- ► 12/14 - 12/21 (10)
- ► 12/21 - 12/28 (3)
-
►
2009
(44)
- ► 01/18 - 01/25 (2)
- ► 02/08 - 02/15 (5)
- ► 03/22 - 03/29 (2)
- ► 03/29 - 04/05 (1)
- ► 04/12 - 04/19 (1)
- ► 05/03 - 05/10 (8)
- ► 05/10 - 05/17 (1)
- ► 05/24 - 05/31 (17)
- ► 07/26 - 08/02 (1)
- ► 10/25 - 11/01 (3)
- ► 12/20 - 12/27 (3)
-
►
2010
(16)
- ► 01/17 - 01/24 (1)
- ► 01/24 - 01/31 (4)
- ► 01/31 - 02/07 (3)
- ► 05/30 - 06/06 (6)
- ► 08/29 - 09/05 (1)
- ► 10/03 - 10/10 (1)
-
►
2011
(21)
- ► 05/01 - 05/08 (11)
- ► 05/15 - 05/22 (10)
Hi ! I AM A SCIENCE TEACHER.

- savitadhutti
- Delhi, Delhi, India
- I AM A SCIENCE TEACHER IN A WELL REPUTED SCHOOL IN INDIA. I AM A MEMBER OF e-shiksha CLUB. I HAVE JOINED TEACHERTUBE FORUM AS WELL. I AM EXPLORING NET TO LEARN AND SHARE.
Nature and you
MOLECULES IN NATURE FORM BONDS JUST LIKE YOU ARE BONDED TO YOUR FRIENDS.


















