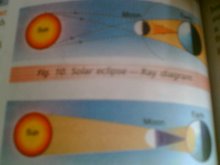
Saturday, 27 December 2008
Force Definition:
The product of mass (m) and acceleration (a). Newton's law of acceleration is used to derive the units of force. With the formula F = Ma in the SI system, one newton is the force needed to accelerate one kilogram of mass by one metre per square second.
Sunday, 14 December 2008
How energy is measured
One of the basic measuring blocks for energy is called a Btu or British thermal unit. Btu is defined as the amount of heat energy it takes to raise the temperature of 1 pound of water by 1 degree Fahrenheit, at sea level. One Btu equals about one black-tip kitchen match. It takes about 2000 Btu to make a pot of coffee.
Energy can also be measured in joules (pronounced the same way as ‘ jewels’). One joule is the amount of energy needed to lift 1 pound about 9 inches. It takes 1000 joules to equal a Btu. It would take 2 million joules to make a pot of coffee.
Joule is named after an English physicist named James Prescott Joule who lived from 1818 to 1889. He discovered that heat is a type of energy.
Around the world, scientists measure energy in joules rather than Btu. It is much like people around the world using the metric system, metres and kilograms. Like in the metric system, you can have kilojoules: ‘kilo’ means 1000, therefore, 1000 joules = 1 kilojoule = 1 Btu.
Energy can also be measured in joules (pronounced the same way as ‘ jewels’). One joule is the amount of energy needed to lift 1 pound about 9 inches. It takes 1000 joules to equal a Btu. It would take 2 million joules to make a pot of coffee.
Joule is named after an English physicist named James Prescott Joule who lived from 1818 to 1889. He discovered that heat is a type of energy.
Around the world, scientists measure energy in joules rather than Btu. It is much like people around the world using the metric system, metres and kilograms. Like in the metric system, you can have kilojoules: ‘kilo’ means 1000, therefore, 1000 joules = 1 kilojoule = 1 Btu.
Due to the problems associated with the use of fossil fuels, alternative sources of energy have become important and relevant in today’s world. These sources, such as the sun and wind, can never be exhausted and are therefore called renewable. Also known as non-conventional sources of energy, they cause less emission and are available locally. Their use can significantly reduce chemical, radioactive, and thermal pollution. They are viable sources of clean and limitless energy. Most of the renewable sources of energy are fairly non-polluting and considered clean. However, biomass is a major polluter indoors.
Renewable energy sources include the sun, wind, water, agricultural residue, fuelwood, and animal dung. Fossil fuels are non-renewable sources. Energy generated from the sun is known as solar energy. Hydel is the energy derived from water. Biomass – firewood, animal dung, and biodegradable waste from cities and crop residues – is a source of energy when it is burnt. Geothermal energy is derived from hot dry rocks, magma, hot water springs, natural geysers, etc. Ocean thermal is energy derived from waves and also from tidal waves.
Through the method of co-generation a cleaner and less polluting form of energy is being generated. Fuel cells are also being used as cleaner energy source. In India a number of initiatives have been taken. A good example is the model village of Ralegaon Siddhi.
Ralegaon Siddhi, a success story –
In 1975, when Anna Hazare, a retired army man, went back to his village in Ahmednagar district, Maharashtra, he found the village reeling under drought, poverty, debt, and unemployment. He decided to mobilize the people and, with the collective support of all the villagers, he began to introduce changes.
Today Ralegaon Siddhi is being taken as a role model for other villages by the Maharashtra government and by other states too. Massive tree plantation has been undertaken, and hills have been terraced to check erosion. Large canals with ridges on either side have been dug to retain rainwater. As a result, the water table in this area is now considerably higher and the wells and tube wells are never dry, making it possible to raise three crops a year where only one was possible before.
The village's biggest achievement is undoubtedly in the area of non-conventional energy. All the streets in the village are lit by solar lights, each with a separate panel. There are four large community biogas plants and one of them is fitted to the community toilet. There is a large windmill used for pumping water. A number of households have their own biogas plants. The village is self sufficient .
types of energy
The discovery of fire by man led to the possibility of burning wood for cooking and heating thereby using energy. For several thousand years human energy demands were met only by renewable energy sources—sun, biomass (wood, leaves, twigs), hydel (water) and wind power.
As early as 4000–3500 BC, the first sailing ships and windmills were developed harnessing wind energy. With the use of hydropower through water mills or irrigation systems, things began to move faster. Fuelwood and dung cakes are even today a major source of energy in rural India. Solar energy is used for drying and heating.
With the advent of the Industrial Revolution, the use of energy in the form of fossil fuels began growing as more and more industries were set up. This occurred in stages, from the exploitation of coal deposits to the exploitation of oil and natural gas fields. It has been only half a century since nuclear power began being used as an energy source. In the past century, it became evident that the consumption of non-renewable sources of energy had caused more environmental damage than any other human activity. Electricity generated from fossil fuels such as coal and crude oil has led to high concentrations of harmful gases in the atmosphere. This has in turn led to problems such as ozone depletion and global warming. Vehicular pollution is also a grave problem.
There has been an enormous increase in the demand for energy since the middle of the last century as a result of industrial development and population growth. World population grew 3.2 times between 1850 and 1970, per capita use of industrial energy increased about twentyfold, and total world use of industrial and traditional energy forms combined increased more than twelvefold.
ENERGY
Can you imagine life without lights, fans, cars, computers and television, or of fetching water from the well and river? This is what life would have been like had man not discovered the uses of energy – both renewable and nonrenewable sources.
What is energy ?
Energy lights our cities, powers our vehicles, and runs machinery in factories. It warms and cools our homes, cooks our food, plays our music, and gives us pictures on television.
Energy is defined as the ability or the capacity to do work.
We use energy to do work and make all movements. When we eat, our bodies transform the food into energy to do work. When we run or walk or do some work, we ‘burn’ energy in our bodies. Cars, planes, trolleys, boats, and machinery also transform energy into work. Work means moving or lifting something, warming or lighting something. There are many sources of energy that help to run the various machines invented by man.
What is energy ?
Energy lights our cities, powers our vehicles, and runs machinery in factories. It warms and cools our homes, cooks our food, plays our music, and gives us pictures on television.
Energy is defined as the ability or the capacity to do work.
We use energy to do work and make all movements. When we eat, our bodies transform the food into energy to do work. When we run or walk or do some work, we ‘burn’ energy in our bodies. Cars, planes, trolleys, boats, and machinery also transform energy into work. Work means moving or lifting something, warming or lighting something. There are many sources of energy that help to run the various machines invented by man.
Quiz time
Statement
A book falls off a table and free falls to the ground.
Answer with Explanation
Yes.
This is an example of work. There is a force (gravity) which acts on the book which causes it to be displaced in a downward direction (i.e., "fall").
A book falls off a table and free falls to the ground.
Answer with Explanation
Yes.
This is an example of work. There is a force (gravity) which acts on the book which causes it to be displaced in a downward direction (i.e., "fall").
work ......concept
When a force acts upon an object to cause a displacement of the object, it is said that work was done upon the object. There are three key ingredients to work - force, displacement, and cause. In order for a force to qualify as having done work on an object, there must be a displacement and the force must cause the displacement. There are several good examples of work which can be observed in everyday life - a horse pulling a plow through the field, a father pushing a grocery cart down the aisle of a grocery store, a freshman lifting a backpack full of books upon her shoulder, a weightlifter lifting a barbell above his head, an Olympian launching the shot-put, etc. In each case described here there is a force exerted upon an object to cause that object to be displaced.
Wednesday, 3 December 2008
Saturday, 29 November 2008
electricity
The three fundamental particles comprising most atoms are called protons, neutrons and electrons. Whilst the majority of atoms have a combination of protons, neutrons, and electrons, not all atoms have neutrons; an example is the protium isotope (1H1) of hydrogen (Hydrogen-1) which is the lightest and most common form of hydrogen which only has one proton and one electron. Atoms are far too small to be seen, but if we could look at one, it might appear something like this:
Even though each atom in a piece of material tends to hold together as a unit, there's actually a lot of empty space between the electrons and the cluster of protons and neutrons residing in the middle.
This crude model is that of the element carbon, with six protons, six neutrons, and six electrons. In any atom, the protons and neutrons are very tightly bound together, which is an important quality. The tightly-bound clump of protons and neutrons in the center of the atom is called the nucleus, and the number of protons in an atom's nucleus determines its elemental identity: change the number of protons in an atom's nucleus, and you change the type of atom that it is. In fact, if you could remove three protons from the nucleus of an atom of lead, you will have achieved the old alchemists' dream of producing an atom of gold! The tight binding of protons in the nucleus is responsible for the stable identity of chemical elements, and the failure of alchemists to achieve their dream.
Neutrons are much less influential on the chemical character and identity of an atom than protons, although they are just as hard to add to or remove from the nucleus, being so tightly bound. If neutrons are added or gained, the atom will still retain the same chemical identity, but its mass will change slightly and it may acquire strange nuclear properties such as radioactivity.
However, electrons have significantly more freedom to move around in an atom than either protons or neutrons. In fact, they can be knocked out of their respective positions (even leaving the atom entirely!) by far less energy than what it takes to dislodge particles in the nucleus. If this happens, the atom still retains its chemical identity, but an important imbalance occurs. Electrons and protons are unique in the fact that they are attracted to one another over a distance. It is this attraction over distance which causes the attraction between rubbed objects, where electrons are moved away from their original atoms to reside around atoms of another object.
Electrons tend to repel other electrons over a distance, as do protons with other protons. The only reason protons bind together in the nucleus of an atom is because of a much stronger force called the strong nuclear force which has effect only under very short distances. Because of this attraction/repulsion behavior between individual particles, electrons and protons are said to have opposite electric charges. That is, each electron has a negative charge, and each proton a positive charge. In equal numbers within an atom, they counteract each other's presence so that the net charge within the atom is zero. This is why the picture of a carbon atom had six electrons: to balance out the electric charge of the six protons in the nucleus. If electrons leave or extra electrons arrive, the atom's net electric charge will be imbalanced, leaving the atom "charged" as a whole, causing it to interact with charged particles and other charged atoms nearby. Neutrons are neither attracted to or repelled by electrons, protons, or even other neutrons, and are consequently categorized as having no charge at all.
The process of electrons arriving or leaving is exactly what happens when certain combinations of materials are rubbed together: electrons from the atoms of one material are forced by the rubbing to leave their respective atoms and transfer over to the atoms of the other material. In other words, electrons comprise the "fluid" hypothesized by Benjamin Franklin. The operational definition of a coulomb as the unit of electrical charge (in terms of force generated between point charges) was found to be equal to an excess or deficiency of about 6,250,000,000,000,000,000 electrons. Or, stated in reverse terms, one electron has a charge of about 0.00000000000000000016 coulombs. Being that one electron is the smallest known carrier of electric charge, this last figure of charge for the electron is defined as the elementary charge.
The result of an imbalance of this "fluid" (electrons) between objects is called static electricity. It is called "static" because the displaced electrons tend to remain stationary after being moved from one insulating material to another. In the case of wax and wool, it was determined through further experimentation that electrons in the wool actually transferred to the atoms in the wax, which is exactly opposite of Franklin's conjecture! In honor of Franklin's designation of the wax's charge being "negative" and the wool's charge being "positive," electrons are said to have a "negative" charging influence. Thus, an object whose atoms have received a surplus of electrons is said to be negatively charged, while an object whose atoms are lacking electrons is said to be positively charged, as confusing as these designations may seem. By the time the true nature of electric "fluid" was discovered, Franklin's nomenclature of electric charge was too well established to be easily changed, and so it remains to this day.
Michael Faraday proved (1832) that static electricity was the same as that produced by a battery or a generator. Static electricity is, for the most part, a nusiance. Black powder and smokeless powder have graphite added to prevent ignition due to static electricity. It causes damage to sensitive semiconductor circuitry. While is is possible to produce motors powered by high voltage and low current characteristic of static electricity, this is not economic. The few practical applications of static electricity include xerographic printing, the electrostatic air filter, and the high voltage Van de Graaff generator.
Even though each atom in a piece of material tends to hold together as a unit, there's actually a lot of empty space between the electrons and the cluster of protons and neutrons residing in the middle.
This crude model is that of the element carbon, with six protons, six neutrons, and six electrons. In any atom, the protons and neutrons are very tightly bound together, which is an important quality. The tightly-bound clump of protons and neutrons in the center of the atom is called the nucleus, and the number of protons in an atom's nucleus determines its elemental identity: change the number of protons in an atom's nucleus, and you change the type of atom that it is. In fact, if you could remove three protons from the nucleus of an atom of lead, you will have achieved the old alchemists' dream of producing an atom of gold! The tight binding of protons in the nucleus is responsible for the stable identity of chemical elements, and the failure of alchemists to achieve their dream.
Neutrons are much less influential on the chemical character and identity of an atom than protons, although they are just as hard to add to or remove from the nucleus, being so tightly bound. If neutrons are added or gained, the atom will still retain the same chemical identity, but its mass will change slightly and it may acquire strange nuclear properties such as radioactivity.
However, electrons have significantly more freedom to move around in an atom than either protons or neutrons. In fact, they can be knocked out of their respective positions (even leaving the atom entirely!) by far less energy than what it takes to dislodge particles in the nucleus. If this happens, the atom still retains its chemical identity, but an important imbalance occurs. Electrons and protons are unique in the fact that they are attracted to one another over a distance. It is this attraction over distance which causes the attraction between rubbed objects, where electrons are moved away from their original atoms to reside around atoms of another object.
Electrons tend to repel other electrons over a distance, as do protons with other protons. The only reason protons bind together in the nucleus of an atom is because of a much stronger force called the strong nuclear force which has effect only under very short distances. Because of this attraction/repulsion behavior between individual particles, electrons and protons are said to have opposite electric charges. That is, each electron has a negative charge, and each proton a positive charge. In equal numbers within an atom, they counteract each other's presence so that the net charge within the atom is zero. This is why the picture of a carbon atom had six electrons: to balance out the electric charge of the six protons in the nucleus. If electrons leave or extra electrons arrive, the atom's net electric charge will be imbalanced, leaving the atom "charged" as a whole, causing it to interact with charged particles and other charged atoms nearby. Neutrons are neither attracted to or repelled by electrons, protons, or even other neutrons, and are consequently categorized as having no charge at all.
The process of electrons arriving or leaving is exactly what happens when certain combinations of materials are rubbed together: electrons from the atoms of one material are forced by the rubbing to leave their respective atoms and transfer over to the atoms of the other material. In other words, electrons comprise the "fluid" hypothesized by Benjamin Franklin. The operational definition of a coulomb as the unit of electrical charge (in terms of force generated between point charges) was found to be equal to an excess or deficiency of about 6,250,000,000,000,000,000 electrons. Or, stated in reverse terms, one electron has a charge of about 0.00000000000000000016 coulombs. Being that one electron is the smallest known carrier of electric charge, this last figure of charge for the electron is defined as the elementary charge.
The result of an imbalance of this "fluid" (electrons) between objects is called static electricity. It is called "static" because the displaced electrons tend to remain stationary after being moved from one insulating material to another. In the case of wax and wool, it was determined through further experimentation that electrons in the wool actually transferred to the atoms in the wax, which is exactly opposite of Franklin's conjecture! In honor of Franklin's designation of the wax's charge being "negative" and the wool's charge being "positive," electrons are said to have a "negative" charging influence. Thus, an object whose atoms have received a surplus of electrons is said to be negatively charged, while an object whose atoms are lacking electrons is said to be positively charged, as confusing as these designations may seem. By the time the true nature of electric "fluid" was discovered, Franklin's nomenclature of electric charge was too well established to be easily changed, and so it remains to this day.
Michael Faraday proved (1832) that static electricity was the same as that produced by a battery or a generator. Static electricity is, for the most part, a nusiance. Black powder and smokeless powder have graphite added to prevent ignition due to static electricity. It causes damage to sensitive semiconductor circuitry. While is is possible to produce motors powered by high voltage and low current characteristic of static electricity, this is not economic. The few practical applications of static electricity include xerographic printing, the electrostatic air filter, and the high voltage Van de Graaff generator.
Static electricity
Glass and silk aren't the only materials known to behave like this. Anyone who has ever brushed up against a latex balloon only to find that it tries to stick to them has experienced this same phenomenon. Paraffin wax and wool cloth are another pair of materials early experimenters recognized as manifesting attractive forces after being rubbed together:
This phenomenon became even more interesting when it was discovered that identical materials, after having been rubbed with their respective cloths, always repelled each other:
It was also noted that when a piece of glass rubbed with silk was exposed to a piece of wax rubbed with wool, the two materials would attract one another:
Furthermore, it was found that any material demonstrating properties of attraction or repulsion after being rubbed could be classed into one of two distinct categories: attracted to glass and repelled by wax, or repelled by glass and attracted to wax. It was either one or the other: there were no materials found that would be attracted to or repelled by both glass and wax, or that reacted to one without reacting to the other.
More attention was directed toward the pieces of cloth used to do the rubbing. It was discovered that after rubbing two pieces of glass with two pieces of silk cloth, not only did the glass pieces repel each other, but so did the cloths. The same phenomenon held for the pieces of wool used to rub the wax:
Now, this was really strange to witness. After all, none of these objects were visibly altered by the rubbing, yet they definitely behaved differently than before they were rubbed. Whatever change took place to make these materials attract or repel one another was invisible.
Some experimenters speculated that invisible "fluids" were being transferred from one object to another during the process of rubbing, and that these "fluids" were able to effect a physical force over a distance. Charles Dufay was one the early experimenters who demonstrated that there were definitely two different types of changes wrought by rubbing certain pairs of objects together. The fact that there was more than one type of change manifested in these materials was evident by the fact that there were two types of forces produced: attraction and repulsion. The hypothetical fluid transfer became known as a charge.
One pioneering researcher, Benjamin Franklin, came to the conclusion that there was only one fluid exchanged between rubbed objects, and that the two different "charges" were nothing more than either an excess or a deficiency of that one fluid. After experimenting with wax and wool, Franklin suggested that the coarse wool removed some of this invisible fluid from the smooth wax, causing an excess of fluid on the wool and a deficiency of fluid on the wax. The resulting disparity in fluid content between the wool and wax would then cause an attractive force, as the fluid tried to regain its former balance between the two materials.
Postulating the existence of a single "fluid" that was either gained or lost through rubbing accounted best for the observed behavior: that all these materials fell neatly into one of two categories when rubbed, and most importantly, that the two active materials rubbed against each other always fell into opposing categories as evidenced by their invariable attraction to one another. In other words, there was never a time where two materials rubbed against each other both became either positive or negative.
Following Franklin's speculation of the wool rubbing something off of the wax, the type of charge that was associated with rubbed wax became known as "negative" (because it was supposed to have a deficiency of fluid) while the type of charge associated with the rubbing wool became known as "positive" (because it was supposed to have an excess of fluid). Little did he know that his innocent conjecture would cause much confusion for students of electricity in the future!
Precise measurements of electrical charge were carried out by the French physicist Charles Coulomb in the 1780's using a device called a torsional balance measuring the force generated between two electrically charged objects. The results of Coulomb's work led to the development of a unit of electrical charge named in his honor, the coulomb. If two "point" objects (hypothetical objects having no appreciable surface area) were equally charged to a measure of 1 coulomb, and placed 1 meter (approximately 1 yard) apart, they would generate a force of about 9 billion newtons (approximately 2 billion pounds), either attracting or repelling depending on the types of charges involved.
It was discovered much later that this "fluid" was actually composed of extremely small bits of matter called electrons, so named in honor of the ancient Greek word for amber: another material exhibiting charged properties when rubbed with cloth. Experimentation has since revealed that all objects are composed of extremely small "building-blocks" known as atoms, and that these atoms are in turn composed of smaller components known as particles. The three fundamental particles comprising most atoms are called protons, neutrons and electrons.
Now, this was really strange to witness. After all, none of these objects were visibly altered by the rubbing, yet they definitely behaved differently than before they were rubbed. Whatever change took place to make these materials attract or repel one another was invisible.
Some experimenters speculated that invisible "fluids" were being transferred from one object to another during the process of rubbing, and that these "fluids" were able to effect a physical force over a distance. Charles Dufay was one the early experimenters who demonstrated that there were definitely two different types of changes wrought by rubbing certain pairs of objects together. The fact that there was more than one type of change manifested in these materials was evident by the fact that there were two types of forces produced: attraction and repulsion. The hypothetical fluid transfer became known as a charge.
One pioneering researcher, Benjamin Franklin, came to the conclusion that there was only one fluid exchanged between rubbed objects, and that the two different "charges" were nothing more than either an excess or a deficiency of that one fluid. After experimenting with wax and wool, Franklin suggested that the coarse wool removed some of this invisible fluid from the smooth wax, causing an excess of fluid on the wool and a deficiency of fluid on the wax. The resulting disparity in fluid content between the wool and wax would then cause an attractive force, as the fluid tried to regain its former balance between the two materials.
Postulating the existence of a single "fluid" that was either gained or lost through rubbing accounted best for the observed behavior: that all these materials fell neatly into one of two categories when rubbed, and most importantly, that the two active materials rubbed against each other always fell into opposing categories as evidenced by their invariable attraction to one another. In other words, there was never a time where two materials rubbed against each other both became either positive or negative.
Following Franklin's speculation of the wool rubbing something off of the wax, the type of charge that was associated with rubbed wax became known as "negative" (because it was supposed to have a deficiency of fluid) while the type of charge associated with the rubbing wool became known as "positive" (because it was supposed to have an excess of fluid). Little did he know that his innocent conjecture would cause much confusion for students of electricity in the future!
Precise measurements of electrical charge were carried out by the French physicist Charles Coulomb in the 1780's using a device called a torsional balance measuring the force generated between two electrically charged objects. The results of Coulomb's work led to the development of a unit of electrical charge named in his honor, the coulomb. If two "point" objects (hypothetical objects having no appreciable surface area) were equally charged to a measure of 1 coulomb, and placed 1 meter (approximately 1 yard) apart, they would generate a force of about 9 billion newtons (approximately 2 billion pounds), either attracting or repelling depending on the types of charges involved.
It was discovered much later that this "fluid" was actually composed of extremely small bits of matter called electrons, so named in honor of the ancient Greek word for amber: another material exhibiting charged properties when rubbed with cloth. Experimentation has since revealed that all objects are composed of extremely small "building-blocks" known as atoms, and that these atoms are in turn composed of smaller components known as particles. The three fundamental particles comprising most atoms are called protons, neutrons and electrons.
Now, this was really strange to witness. After all, none of these objects were visibly altered by the rubbing, yet they definitely behaved differently than before they were rubbed. Whatever change took place to make these materials attract or repel one another was invisible.
Some experimenters speculated that invisible "fluids" were being transferred from one object to another during the process of rubbing, and that these "fluids" were able to effect a physical force over a distance. Charles Dufay was one the early experimenters who demonstrated that there were definitely two different types of changes wrought by rubbing certain pairs of objects together. The fact that there was more than one type of change manifested in these materials was evident by the fact that there were two types of forces produced: attraction and repulsion. The hypothetical fluid transfer became known as a charge.
One pioneering researcher, Benjamin Franklin, came to the conclusion that there was only one fluid exchanged between rubbed objects, and that the two different "charges" were nothing more than either an excess or a deficiency of that one fluid. After experimenting with wax and wool, Franklin suggested that the coarse wool removed some of this invisible fluid from the smooth wax, causing an excess of fluid on the wool and a deficiency of fluid on the wax. The resulting disparity in fluid content between the wool and wax would then cause an attractive force, as the fluid tried to regain its former balance between the two materials.
Postulating the existence of a single "fluid" that was either gained or lost through rubbing accounted best for the observed behavior: that all these materials fell neatly into one of two categories when rubbed, and most importantly, that the two active materials rubbed against each other always fell into opposing categories as evidenced by their invariable attraction to one another. In other words, there was never a time where two materials rubbed against each other both became either positive or negative.
Following Franklin's speculation of the wool rubbing something off of the wax, the type of charge that was associated with rubbed wax became known as "negative" (because it was supposed to have a deficiency of fluid) while the type of charge associated with the rubbing wool became known as "positive" (because it was supposed to have an excess of fluid). Little did he know that his innocent conjecture would cause much confusion for students of electricity in the future!
Precise measurements of electrical charge were carried out by the French physicist Charles Coulomb in the 1780's using a device called a torsional balance measuring the force generated between two electrically charged objects. The results of Coulomb's work led to the development of a unit of electrical charge named in his honor, the coulomb. If two "point" objects (hypothetical objects having no appreciable surface area) were equally charged to a measure of 1 coulomb, and placed 1 meter (approximately 1 yard) apart, they would generate a force of about 9 billion newtons (approximately 2 billion pounds), either attracting or repelling depending on the types of charges involved.
It was discovered much later that this "fluid" was actually composed of extremely small bits of matter called electrons, so named in honor of the ancient Greek word for amber: another material exhibiting charged properties when rubbed with cloth. Experimentation has since revealed that all objects are composed of extremely small "building-blocks" known as atoms, and that these atoms are in turn composed of smaller components known as particles. The three fundamental particles comprising most atoms are called protons, neutrons and electrons.
This phenomenon became even more interesting when it was discovered that identical materials, after having been rubbed with their respective cloths, always repelled each other:
It was also noted that when a piece of glass rubbed with silk was exposed to a piece of wax rubbed with wool, the two materials would attract one another:
Furthermore, it was found that any material demonstrating properties of attraction or repulsion after being rubbed could be classed into one of two distinct categories: attracted to glass and repelled by wax, or repelled by glass and attracted to wax. It was either one or the other: there were no materials found that would be attracted to or repelled by both glass and wax, or that reacted to one without reacting to the other.
More attention was directed toward the pieces of cloth used to do the rubbing. It was discovered that after rubbing two pieces of glass with two pieces of silk cloth, not only did the glass pieces repel each other, but so did the cloths. The same phenomenon held for the pieces of wool used to rub the wax:
Now, this was really strange to witness. After all, none of these objects were visibly altered by the rubbing, yet they definitely behaved differently than before they were rubbed. Whatever change took place to make these materials attract or repel one another was invisible.
Some experimenters speculated that invisible "fluids" were being transferred from one object to another during the process of rubbing, and that these "fluids" were able to effect a physical force over a distance. Charles Dufay was one the early experimenters who demonstrated that there were definitely two different types of changes wrought by rubbing certain pairs of objects together. The fact that there was more than one type of change manifested in these materials was evident by the fact that there were two types of forces produced: attraction and repulsion. The hypothetical fluid transfer became known as a charge.
One pioneering researcher, Benjamin Franklin, came to the conclusion that there was only one fluid exchanged between rubbed objects, and that the two different "charges" were nothing more than either an excess or a deficiency of that one fluid. After experimenting with wax and wool, Franklin suggested that the coarse wool removed some of this invisible fluid from the smooth wax, causing an excess of fluid on the wool and a deficiency of fluid on the wax. The resulting disparity in fluid content between the wool and wax would then cause an attractive force, as the fluid tried to regain its former balance between the two materials.
Postulating the existence of a single "fluid" that was either gained or lost through rubbing accounted best for the observed behavior: that all these materials fell neatly into one of two categories when rubbed, and most importantly, that the two active materials rubbed against each other always fell into opposing categories as evidenced by their invariable attraction to one another. In other words, there was never a time where two materials rubbed against each other both became either positive or negative.
Following Franklin's speculation of the wool rubbing something off of the wax, the type of charge that was associated with rubbed wax became known as "negative" (because it was supposed to have a deficiency of fluid) while the type of charge associated with the rubbing wool became known as "positive" (because it was supposed to have an excess of fluid). Little did he know that his innocent conjecture would cause much confusion for students of electricity in the future!
Precise measurements of electrical charge were carried out by the French physicist Charles Coulomb in the 1780's using a device called a torsional balance measuring the force generated between two electrically charged objects. The results of Coulomb's work led to the development of a unit of electrical charge named in his honor, the coulomb. If two "point" objects (hypothetical objects having no appreciable surface area) were equally charged to a measure of 1 coulomb, and placed 1 meter (approximately 1 yard) apart, they would generate a force of about 9 billion newtons (approximately 2 billion pounds), either attracting or repelling depending on the types of charges involved.
It was discovered much later that this "fluid" was actually composed of extremely small bits of matter called electrons, so named in honor of the ancient Greek word for amber: another material exhibiting charged properties when rubbed with cloth. Experimentation has since revealed that all objects are composed of extremely small "building-blocks" known as atoms, and that these atoms are in turn composed of smaller components known as particles. The three fundamental particles comprising most atoms are called protons, neutrons and electrons.
Now, this was really strange to witness. After all, none of these objects were visibly altered by the rubbing, yet they definitely behaved differently than before they were rubbed. Whatever change took place to make these materials attract or repel one another was invisible.
Some experimenters speculated that invisible "fluids" were being transferred from one object to another during the process of rubbing, and that these "fluids" were able to effect a physical force over a distance. Charles Dufay was one the early experimenters who demonstrated that there were definitely two different types of changes wrought by rubbing certain pairs of objects together. The fact that there was more than one type of change manifested in these materials was evident by the fact that there were two types of forces produced: attraction and repulsion. The hypothetical fluid transfer became known as a charge.
One pioneering researcher, Benjamin Franklin, came to the conclusion that there was only one fluid exchanged between rubbed objects, and that the two different "charges" were nothing more than either an excess or a deficiency of that one fluid. After experimenting with wax and wool, Franklin suggested that the coarse wool removed some of this invisible fluid from the smooth wax, causing an excess of fluid on the wool and a deficiency of fluid on the wax. The resulting disparity in fluid content between the wool and wax would then cause an attractive force, as the fluid tried to regain its former balance between the two materials.
Postulating the existence of a single "fluid" that was either gained or lost through rubbing accounted best for the observed behavior: that all these materials fell neatly into one of two categories when rubbed, and most importantly, that the two active materials rubbed against each other always fell into opposing categories as evidenced by their invariable attraction to one another. In other words, there was never a time where two materials rubbed against each other both became either positive or negative.
Following Franklin's speculation of the wool rubbing something off of the wax, the type of charge that was associated with rubbed wax became known as "negative" (because it was supposed to have a deficiency of fluid) while the type of charge associated with the rubbing wool became known as "positive" (because it was supposed to have an excess of fluid). Little did he know that his innocent conjecture would cause much confusion for students of electricity in the future!
Precise measurements of electrical charge were carried out by the French physicist Charles Coulomb in the 1780's using a device called a torsional balance measuring the force generated between two electrically charged objects. The results of Coulomb's work led to the development of a unit of electrical charge named in his honor, the coulomb. If two "point" objects (hypothetical objects having no appreciable surface area) were equally charged to a measure of 1 coulomb, and placed 1 meter (approximately 1 yard) apart, they would generate a force of about 9 billion newtons (approximately 2 billion pounds), either attracting or repelling depending on the types of charges involved.
It was discovered much later that this "fluid" was actually composed of extremely small bits of matter called electrons, so named in honor of the ancient Greek word for amber: another material exhibiting charged properties when rubbed with cloth. Experimentation has since revealed that all objects are composed of extremely small "building-blocks" known as atoms, and that these atoms are in turn composed of smaller components known as particles. The three fundamental particles comprising most atoms are called protons, neutrons and electrons.
Now, this was really strange to witness. After all, none of these objects were visibly altered by the rubbing, yet they definitely behaved differently than before they were rubbed. Whatever change took place to make these materials attract or repel one another was invisible.
Some experimenters speculated that invisible "fluids" were being transferred from one object to another during the process of rubbing, and that these "fluids" were able to effect a physical force over a distance. Charles Dufay was one the early experimenters who demonstrated that there were definitely two different types of changes wrought by rubbing certain pairs of objects together. The fact that there was more than one type of change manifested in these materials was evident by the fact that there were two types of forces produced: attraction and repulsion. The hypothetical fluid transfer became known as a charge.
One pioneering researcher, Benjamin Franklin, came to the conclusion that there was only one fluid exchanged between rubbed objects, and that the two different "charges" were nothing more than either an excess or a deficiency of that one fluid. After experimenting with wax and wool, Franklin suggested that the coarse wool removed some of this invisible fluid from the smooth wax, causing an excess of fluid on the wool and a deficiency of fluid on the wax. The resulting disparity in fluid content between the wool and wax would then cause an attractive force, as the fluid tried to regain its former balance between the two materials.
Postulating the existence of a single "fluid" that was either gained or lost through rubbing accounted best for the observed behavior: that all these materials fell neatly into one of two categories when rubbed, and most importantly, that the two active materials rubbed against each other always fell into opposing categories as evidenced by their invariable attraction to one another. In other words, there was never a time where two materials rubbed against each other both became either positive or negative.
Following Franklin's speculation of the wool rubbing something off of the wax, the type of charge that was associated with rubbed wax became known as "negative" (because it was supposed to have a deficiency of fluid) while the type of charge associated with the rubbing wool became known as "positive" (because it was supposed to have an excess of fluid). Little did he know that his innocent conjecture would cause much confusion for students of electricity in the future!
Precise measurements of electrical charge were carried out by the French physicist Charles Coulomb in the 1780's using a device called a torsional balance measuring the force generated between two electrically charged objects. The results of Coulomb's work led to the development of a unit of electrical charge named in his honor, the coulomb. If two "point" objects (hypothetical objects having no appreciable surface area) were equally charged to a measure of 1 coulomb, and placed 1 meter (approximately 1 yard) apart, they would generate a force of about 9 billion newtons (approximately 2 billion pounds), either attracting or repelling depending on the types of charges involved.
It was discovered much later that this "fluid" was actually composed of extremely small bits of matter called electrons, so named in honor of the ancient Greek word for amber: another material exhibiting charged properties when rubbed with cloth. Experimentation has since revealed that all objects are composed of extremely small "building-blocks" known as atoms, and that these atoms are in turn composed of smaller components known as particles. The three fundamental particles comprising most atoms are called protons, neutrons and electrons.
Static electricity
It was discovered centuries ago that certain types of materials would mysteriously attract one another after being rubbed together. For example: after rubbing a piece of silk against a piece of glass, the silk and glass would tend to stick together. Indeed, there was an attractive force that could be demonstrated even when the two materials were separated:
Electric circuits
You might have been wondering how electrons can continuously flow in a uniform direction through wires without the benefit of these hypothetical electron Sources and Destinations. In order for the Source-and-Destination scheme to work, both would have to have an infinite capacity for electrons in order to sustain a continuous flow! Using the marble-and-tube analogy, the marble source and marble destination buckets would have to be infinitely large to contain enough marble capacity for a "flow" of marbles to be sustained.
The answer to this paradox is found in the concept of a circuit: a never-ending looped pathway for electrons. If we take a wire, or many wires joined end-to-end, and loop it around so that it forms a continuous pathway, we have the means to support a uniform flow of electrons without having to resort to infinite Sources and Destinations:
Each electron advancing clockwise in this circuit pushes on the one in front of it, which pushes on the one in front of it, and so on, and so on, just like a hula-hoop filled with marbles. Now, we have the capability of supporting a continuous flow of electrons indefinitely without the need for infinite electron supplies and dumps. All we need to maintain this flow is a continuous means of motivation for those electrons, which we'll address in the next section of this chapter.
It must be realized that continuity is just as important in a circuit as it is in a straight piece of wire. Just as in the example with the straight piece of wire between the electron Source and Destination, any break in this circuit will prevent electrons from flowing through it:
An important principle to realize here is that it doesn't matter where the break occurs. Any discontinuity in the circuit will prevent electron flow throughout the entire circuit. Unless there is a continuous, unbroken loop of conductive material for electrons to flow through, a sustained flow simply cannot be maintained.
The answer to this paradox is found in the concept of a circuit: a never-ending looped pathway for electrons. If we take a wire, or many wires joined end-to-end, and loop it around so that it forms a continuous pathway, we have the means to support a uniform flow of electrons without having to resort to infinite Sources and Destinations:
Each electron advancing clockwise in this circuit pushes on the one in front of it, which pushes on the one in front of it, and so on, and so on, just like a hula-hoop filled with marbles. Now, we have the capability of supporting a continuous flow of electrons indefinitely without the need for infinite electron supplies and dumps. All we need to maintain this flow is a continuous means of motivation for those electrons, which we'll address in the next section of this chapter.
It must be realized that continuity is just as important in a circuit as it is in a straight piece of wire. Just as in the example with the straight piece of wire between the electron Source and Destination, any break in this circuit will prevent electrons from flowing through it:
An important principle to realize here is that it doesn't matter where the break occurs. Any discontinuity in the circuit will prevent electron flow throughout the entire circuit. Unless there is a continuous, unbroken loop of conductive material for electrons to flow through, a sustained flow simply cannot be maintained.
Wednesday, 26 November 2008
Structure of an electric torch
Why did the designer choose this particular combination of materials? The metal parts of the torch must conduct electric current if the torch is to function, but they must also be able to stand up to physical forces. The spring holding the cells in place should stay springy, while the parts of the switch must make good electrical contact and be undamaged by repeated use.
The lamp and reflector make up an optical system, often intended to focus the light into a narrow beam. The plastic casing is an electrical insulator. Its shape and colour are important in making the torch attractive and easy to handle and use.
A torch is a simple product, but a lot of thought is needed to make sure that it will work well. Can you think of other things which the designer should consider?
A different way of describing the torch is by using a circuit diagram in which the parts of the torch are represented by symbols:
There are two electric cells ('batteries'), a switch and a lamp (the torch bulb). The lines in the diagram represent the metal conductors which connect the system together.
A circuit is a closed conducting path. In the torch, closing the switch completes the circuit and allows current to flow. Torches sometimes fail when the metal parts of the switch do no make proper contact, or when the lamp filament is 'blown'. In either case, the circuit is incomplete.
Current
An electric current is a flow of charged particles. Inside a copper wire, current is carried by small negatively-charged particles, called electrons. The electrons drift in random directions until a current starts to flow. When this happens, electrons start to move in the same direction. The size of the current depends on the number of electrons passing per second.
Current is represented by the symbol I, and is measured in amperes, or 'amps', A. One ampere is a flow of 6.24 x 1018 electrons per second past any point in a wire. That's more than six million million million electrons passing per second. This is a lot of electrons, but electrons are very small and each carries a very tiny charge.
In electronic circuits, currents are most often measured in milliamps, mA, that is, thousandths of an amp.
how batteries work
A battery is essentially a can full of chemicals that produce electrons. Chemical reactions that produce electrons are called electrochemical reactions. In this article, you'll learn all about batteries -- the basic concept at work, the actual chemistry going on inside a battery, rechargeable versions, what the future holds for batteries and possible power sources that could replace them.
If you look at any battery, you'll notice that it has two terminals. One terminal is marked (+), or positive, while the other is marked (-), or negative. In an AA, C or D cell (normal flashlight batteries), the ends of the battery are the terminals. In a large car battery, there are two heavy lead posts that act as the terminals.
Electrons collect on the negative terminal of the battery. If you connect a wire between the negative and positive terminals, the electrons will flow from the negative to the positive terminal as fast as they can (and wear out the battery very quickly -- this also tends to be dangerous, especially with large batteries, so it is not something you want to be doing). Normally, you connect some type of load to the battery using the wire.
If you look at any battery, you'll notice that it has two terminals. One terminal is marked (+), or positive, while the other is marked (-), or negative. In an AA, C or D cell (normal flashlight batteries), the ends of the battery are the terminals. In a large car battery, there are two heavy lead posts that act as the terminals.
Electrons collect on the negative terminal of the battery. If you connect a wire between the negative and positive terminals, the electrons will flow from the negative to the positive terminal as fast as they can (and wear out the battery very quickly -- this also tends to be dangerous, especially with large batteries, so it is not something you want to be doing). Normally, you connect some type of load to the battery using the wire.
Saturday, 4 October 2008
Thursday, 2 October 2008
Wednesday, 24 September 2008
Sunday, 14 September 2008
Sunday, 31 August 2008
Saturday, 23 August 2008
Wednesday, 13 August 2008
Monday, 14 July 2008
holidays home work was good
well done students!
I am glad you all have done your holidays home work nicely.
Its time now to prepare for the forthcoming unit tests.
keep visiting the blog for the revision work,kids!
Best of luck!!!
I am glad you all have done your holidays home work nicely.
Its time now to prepare for the forthcoming unit tests.
keep visiting the blog for the revision work,kids!
Best of luck!!!
Tuesday, 1 July 2008
Announcement
Dear students!
Hope you enjoyed surfing the blog and solved the puzzle as instructed.Its the time now to submit the H.HW.The date of submission is 5th of July,2008.
Hope you enjoyed surfing the blog and solved the puzzle as instructed.Its the time now to submit the H.HW.The date of submission is 5th of July,2008.
Saturday, 14 June 2008
How can you celebrate World Environment Day?
World Environment Day can be celebrated in many ways, including street rallies, bicycles parades, green concerts, essay and poster competitions in schools, tree planting, recycling efforts, clean-up campaigns and much more. In many countries, this annual event is used to enhance political attention and action.
Heads of State, Prime Ministers and Ministers of Environment deliver statements and commit themselves to care for the Earth. Serious pledges are made which lead to the establishment of permanent governmental structures dealing with environmental management and economic planning. This observance also provides an opportunity to sign or ratify international environmental conventions.
On this World Environment Day, let us examine the state of our environment. Let us consider carefully the actions which each of us must take, and then address ourselves to our common task of preserving all life on earth in a mood of sober resolution and quiet confidence.
World Environment Day 2007 Tromsø, Norway
Heads of State, Prime Ministers and Ministers of Environment deliver statements and commit themselves to care for the Earth. Serious pledges are made which lead to the establishment of permanent governmental structures dealing with environmental management and economic planning. This observance also provides an opportunity to sign or ratify international environmental conventions.
On this World Environment Day, let us examine the state of our environment. Let us consider carefully the actions which each of us must take, and then address ourselves to our common task of preserving all life on earth in a mood of sober resolution and quiet confidence.
World Environment Day 2007 Tromsø, Norway
WORLD ENVIRONMENT DAY 5 JUNE 2008
World Environment Day, commemorated each year on 5 June, is one of the principal vehicles through which the United Nations stimulates worldwide awareness of the environment and enhances political attention and action.
The World Environment Day slogan for 2008 is Kick the Habit! Towards a Low Carbon Economy. Recognising that climate change is becoming the defining issue of our era, UNEP is asking countries, companies and communities to focus on greenhouse gas emissions and how to reduce them. The World Environment Day will highlight resources and initiatives that promote low carbon economies and life-styles, such as improved energy efficiency, alternative energy sources, forest conservation and eco-friendly consumption.
The main international celebrations of World Environment Day 2008 will be held in New Zealand. UNEP is honoured that the city of Wellington will be hosting this United Nations day (read the press release).
The day's agenda is to give a human face to environmental issues; empower people to become active agents of sustainable and equitable development; promote an understanding that communities are pivotal to changing attitudes towards environmental issues; and advocate partnership, which will ensure all nations and peoples enjoy a safer and more prosperous future.
The World Environment Day slogan for 2008 is Kick the Habit! Towards a Low Carbon Economy. Recognising that climate change is becoming the defining issue of our era, UNEP is asking countries, companies and communities to focus on greenhouse gas emissions and how to reduce them. The World Environment Day will highlight resources and initiatives that promote low carbon economies and life-styles, such as improved energy efficiency, alternative energy sources, forest conservation and eco-friendly consumption.
The main international celebrations of World Environment Day 2008 will be held in New Zealand. UNEP is honoured that the city of Wellington will be hosting this United Nations day (read the press release).
The day's agenda is to give a human face to environmental issues; empower people to become active agents of sustainable and equitable development; promote an understanding that communities are pivotal to changing attitudes towards environmental issues; and advocate partnership, which will ensure all nations and peoples enjoy a safer and more prosperous future.
Host Countries of International World Environment day and Global 500 Ceremony Event
Year City Country
1999 Tokyo Japan
1998 Moscow Russian Federation
1997 Seoul Republic of Korea
1996 Istanbul Turkey
1995 Pretoria South Africa
1994 London United Kingdom
1993 Beijing China
1992 Rio de Janeiro Brazil
1991 Stockholm Sweeden
1990 Maxico City Mexico
1989 Brussels Belgium
1988 Bangkok Thailand
1987 Nairobi Kenya
[
1999 Tokyo Japan
1998 Moscow Russian Federation
1997 Seoul Republic of Korea
1996 Istanbul Turkey
1995 Pretoria South Africa
1994 London United Kingdom
1993 Beijing China
1992 Rio de Janeiro Brazil
1991 Stockholm Sweeden
1990 Maxico City Mexico
1989 Brussels Belgium
1988 Bangkok Thailand
1987 Nairobi Kenya
[
Wed Themes for the Environment day
Year Theme
1999 Our Earth-Our Future-Just Save It!
1998 For Life on Earth - Save Our Seas
1997 For Life on Earth
1996 Our Earth,Our Habitat,Our Home
1995 We the peoples:United for the Global Environment
1994 One Earth One Family
1993 Poverty and the Environment - Breaking the vicious Circle
1992 Only One Earth - Care and Share
1991 Climate Change: Need for Global Partnership
1990 Children and the Environment
1989 Global Warming : Global Warning
1988 When People Put the Environment First, Development Will Last
1987 Environment and Shelter : More Than A Roof
1986 A Tree For Peace
1985 Youth : Population and the Environment
1984 Desertification
1983 Managing and Disposing Hazardous Waste : Acid Rain and Energy
1982 Ten Years After Stockholm (Renewal of Environmental Concerns)
1981 Ground Water; Toxic Chemicals in Human Food Chains and Environmental Economics
1980 A New Challenge for the New Decade:Development Without Destruction
1979 Only One Future for Our Children - Development Without Destruction
1978 Development Without Destruction
1977 Ozone Layer Environmental Concern;Lands Loss and Soil Degradation; Firewood
1976 Water: Vital Resource for Life
1975 Human Settlements
1974 Only one Earth
1999 Our Earth-Our Future-Just Save It!
1998 For Life on Earth - Save Our Seas
1997 For Life on Earth
1996 Our Earth,Our Habitat,Our Home
1995 We the peoples:United for the Global Environment
1994 One Earth One Family
1993 Poverty and the Environment - Breaking the vicious Circle
1992 Only One Earth - Care and Share
1991 Climate Change: Need for Global Partnership
1990 Children and the Environment
1989 Global Warming : Global Warning
1988 When People Put the Environment First, Development Will Last
1987 Environment and Shelter : More Than A Roof
1986 A Tree For Peace
1985 Youth : Population and the Environment
1984 Desertification
1983 Managing and Disposing Hazardous Waste : Acid Rain and Energy
1982 Ten Years After Stockholm (Renewal of Environmental Concerns)
1981 Ground Water; Toxic Chemicals in Human Food Chains and Environmental Economics
1980 A New Challenge for the New Decade:Development Without Destruction
1979 Only One Future for Our Children - Development Without Destruction
1978 Development Without Destruction
1977 Ozone Layer Environmental Concern;Lands Loss and Soil Degradation; Firewood
1976 Water: Vital Resource for Life
1975 Human Settlements
1974 Only one Earth
What Prime Minister, Mr. Atal Behari Vajpayee said.............
Speaking at a function to mark the World Environment Day, Prime Minister, Mr. Atal Behari Vajpayee cautioned that if the rich nations continued to put unrelenting pressure on our planet's limited and non-renewable resources, and if the present glaring imbalances in global economic growth continued, it would be difficult to prevent the damages to the environment on a universal scale.
He regretted that ironically it is the poor who have to pay a heavier price for the guilt of the rich. He called for radical changes in the international financial and trading systems, so as to bridge the gulf between the developing and the developed nations, and to halt the worsening condition of want and suffering in developing nations, which are not only a source of social discord but also of environmental degradation. Stressing that there was no basic conflict between development and environmental protection, the Prime Minister called for broadening and deepening the sweep of environmental protection as a people's movement.
He called WED a day "to focus our attention on our collective failure to protect the environment, which has endangered sustainable development for the human race". He said, "conservation and protection of the environment have been the cornerstone of Indian ethos and culture." He added, "in spite of this cultural tradition, the state of the environment in India today ought to be a cause of deep concern to all of us. Many of our cities are among the most polluted in the world. Our rivers at many places - including in Delhi- have become highly dirty. Our forest cover is rapidly depleting." He pointed out that soil erosion and degradation had become a major problem and was adversely affecting our agriculture. He said, "The amenities for safe drinking water and sanitation are so inadequate for the poor in cities and in villages that they are harming their health and happiness. Already the water table is dropping so fast in many places that we may not have enough water for all our needs in the coming decades."
Advising the industry he said, "I make a fervent appeal today. Don't wait for either the Government or the judiciary to enforce environmental laws. Voluntary and speedy compliance is good for both industry and society.
Do not think of investment in environment- friendly technologies as a burden you can shirk. It is a moral, social, and legal obligation you must fulfil. Moreover, it makes good business sense in the medium and long term. Wherever possible - and it is possible in many cases - we should also implement low cost green technologies that are appropriate to our needs and conditions."
He regretted that ironically it is the poor who have to pay a heavier price for the guilt of the rich. He called for radical changes in the international financial and trading systems, so as to bridge the gulf between the developing and the developed nations, and to halt the worsening condition of want and suffering in developing nations, which are not only a source of social discord but also of environmental degradation. Stressing that there was no basic conflict between development and environmental protection, the Prime Minister called for broadening and deepening the sweep of environmental protection as a people's movement.
He called WED a day "to focus our attention on our collective failure to protect the environment, which has endangered sustainable development for the human race". He said, "conservation and protection of the environment have been the cornerstone of Indian ethos and culture." He added, "in spite of this cultural tradition, the state of the environment in India today ought to be a cause of deep concern to all of us. Many of our cities are among the most polluted in the world. Our rivers at many places - including in Delhi- have become highly dirty. Our forest cover is rapidly depleting." He pointed out that soil erosion and degradation had become a major problem and was adversely affecting our agriculture. He said, "The amenities for safe drinking water and sanitation are so inadequate for the poor in cities and in villages that they are harming their health and happiness. Already the water table is dropping so fast in many places that we may not have enough water for all our needs in the coming decades."
Advising the industry he said, "I make a fervent appeal today. Don't wait for either the Government or the judiciary to enforce environmental laws. Voluntary and speedy compliance is good for both industry and society.
Do not think of investment in environment- friendly technologies as a burden you can shirk. It is a moral, social, and legal obligation you must fulfil. Moreover, it makes good business sense in the medium and long term. Wherever possible - and it is possible in many cases - we should also implement low cost green technologies that are appropriate to our needs and conditions."
World Environment day: 5th June
United Nations General Assembly in 1972 established the World Environment Day. On the same day United Nations Environment Programme (UNEP) was created too.Today World Environment Day is celebrated world over in many ways. World Environment Day (WED) is not just another day but a special day about you and me. Green concerts, essay and poster competitions, rallies, tree planting, recycling efforts, clean-up campaigns etc. are some of the ways people, organisations and the governments express their concern towards the environment. WED is also used to enhance political attention and action. Pledges are made and actions are taken, which lead to the establishment of permanent governmental structures dealing with environmental management and economic planning.
Nuclear Power Corporation (NPC) celebrated WED with enthusiasm. At Tarapur Atomic Power Station, for instance, weeklong celebrations were held. Slogan competition on the theme, "Prevention of Pollution and Protection of Environment", and two lectures on environmental monitoring and impact assessment were organised. The environmental survey laboratory (ESL) was kept open to public. One hundred forty villagers visited the ESL, and were briefed on the activities and facilities at the ESL.
Environmental awareness was the focus at Kaiga Atomic Power Project. The 'Environmental Radiological Laboratory' (ERL), the 'health physics unit' and the 'public relations' wing of the Kaiga Project jointly managed the event. Mr. V. K. Sharma, Project Director of the Kaiga Project, inaugurated the daylong celebration. The main attraction at Kaiga was an exhibition depicting various environmental and nuclear safety aspects. The exhibition and the ERL were open to the members of public. Radioactivity, peaceful uses of atomic energy, environmental sampling programme by the ERL, results of preoperational survey etc. were depicted through charts. photographs, and video shows. Demonstrations of Grab Sampler for the sediment, depth water sampler, plankton net, the use of protective wares and personnel radiation measurement system were given to the visitors. School children enthusiastically participated in 'natural vs. man-made radiation' experiments. Computer aided audio video shows were also arranged for the visitors. About 300 people from the adjoining villages and residents of Kaiga visited the exhibition.
World Environment Day is also an intellectual event. Seminars, roundtable meetings and symposia are organised to pool the intellectual inputs. The World Environment Foundation organised a two-day international conference on "Environment Management: Building Synergy between Business and Environment during June 5-6, 1999 in New Delhi. Mr. M. Das, Chief Engineer (HSE&PA) of NPCIL presented a paper. "Nuclear Energy: Power Source for Sustainable Development in India" in the Congress. The 'Golden Peacock Environment Management Award' was given, during the Congress, to the Rajasthan Atomic Power Station (RAPS) of NPCIL, by the Union Minister for Environment and Forests, Mr. Suresh P. Prabhu, in New Delhi on June 5, 1999 (see Indian News in this issue).
Nuclear Power Corporation (NPC) celebrated WED with enthusiasm. At Tarapur Atomic Power Station, for instance, weeklong celebrations were held. Slogan competition on the theme, "Prevention of Pollution and Protection of Environment", and two lectures on environmental monitoring and impact assessment were organised. The environmental survey laboratory (ESL) was kept open to public. One hundred forty villagers visited the ESL, and were briefed on the activities and facilities at the ESL.
Environmental awareness was the focus at Kaiga Atomic Power Project. The 'Environmental Radiological Laboratory' (ERL), the 'health physics unit' and the 'public relations' wing of the Kaiga Project jointly managed the event. Mr. V. K. Sharma, Project Director of the Kaiga Project, inaugurated the daylong celebration. The main attraction at Kaiga was an exhibition depicting various environmental and nuclear safety aspects. The exhibition and the ERL were open to the members of public. Radioactivity, peaceful uses of atomic energy, environmental sampling programme by the ERL, results of preoperational survey etc. were depicted through charts. photographs, and video shows. Demonstrations of Grab Sampler for the sediment, depth water sampler, plankton net, the use of protective wares and personnel radiation measurement system were given to the visitors. School children enthusiastically participated in 'natural vs. man-made radiation' experiments. Computer aided audio video shows were also arranged for the visitors. About 300 people from the adjoining villages and residents of Kaiga visited the exhibition.
World Environment Day is also an intellectual event. Seminars, roundtable meetings and symposia are organised to pool the intellectual inputs. The World Environment Foundation organised a two-day international conference on "Environment Management: Building Synergy between Business and Environment during June 5-6, 1999 in New Delhi. Mr. M. Das, Chief Engineer (HSE&PA) of NPCIL presented a paper. "Nuclear Energy: Power Source for Sustainable Development in India" in the Congress. The 'Golden Peacock Environment Management Award' was given, during the Congress, to the Rajasthan Atomic Power Station (RAPS) of NPCIL, by the Union Minister for Environment and Forests, Mr. Suresh P. Prabhu, in New Delhi on June 5, 1999 (see Indian News in this issue).
Wednesday, 21 May 2008
Try Other Objects
Try other materials, too, like wood, plastic, carbon, cotton, wool, glass, concrete, leaves, CDs, and so on, which you can find around the house.
Some things which will be attracted to or stick to a very strong magnet, like a rare-earth magnet, is the tape from a VCR or audio tape, a dollar bill, and the surface of a floppy disk. The reason these items will stick to a magnet is because of the very small particles of iron used in the ink of the dollar bill, and the iron oxide (ferric oxide) used as the recording medium for the VCR and audio tapes and for the floppy disk. (Please only use a tape or disk which you want to destroy!)
Let's try an experiment:
As you can see in the photo above, the tape from a VCR is attracted to the rare-earth magnet. The magnet will erase the information contained on that section of the VCR tape. I used a pencil to hold open the flip-top cover.
How about a dollar bill?
On the other two photos, you can see how the bill is attracted to the rare-earth magnet.
Take a crisp bill.
Fold it about 55% of the way along its length.
Lay it on a table as shown with the longer portion on the table, the shorter portion sticking up.
Bring the magnet close to the edge of the bill.
Watch the bill spring toward the magnet.
The reason for the attraction is that the ink on the bill has some iron particles in it.
To see what effect a magnet has on floppy disks:
Take a floppy disk and try these things with it. Be sure to record exactly what you do and your observations - the two most important parts of an experiment!
Be sure to try some typical refrigerator magnets (usually very weak since they can barely hold one piece of paper to the fridge door) as well as some stronger rare-earth magnets (neodymium-iron-boron magnets which can easily hold a stack of 20 sheets to the fridge).
Also, vary how the magnet approaches the floppy disk and leaves the disk.
For example - directly toward it, perpendicular to the plane of the disk,
or across the face of the disk, in parallel to the plane of the disk.
Perhaps a quick approach and a slow approach could also be compared.
Try the top side and the bottom side of the disk.
Even try moving the magnet around in a circle on the face of the disk.
Maybe even have a floppy held to the fridge by a magnet for a week to see if time has any affect.
If you can make an AC electromagnet, that would also be a great addition for comparison.
What kind of data will you put on the disk in order to see if the data has been corrupted?
Perhaps some bitmap images would work well, with a simple pattern of black and white squares. They are usually large files so they would cover a large part of the disk. Also, looking at the image would be a very quick and easy way to determine if any bits were changed.
Another method would be to have a large data file on the disk, and do a file compare to the original which is kept on the hard drive.
Want to try something a bit unusual? You know that several cereals claim to be "iron fortified". How do they do that? By adding some finely powdered iron (like small iron filings) in with the cereal as it is being mixed. To see this, simply do the following:
a. Get some cereal that has a large percentage of the RDA (Recommended Dietary Allowance) for iron, and pour half a serving into a bowl.
b. Add water (no need to waste the milk) to the cereal.
c. Mix up the stuff so that it is a watery slurry, not very thick.
d. Take a strong rare-earth magnet and place it into an inside-out zip-lock bag. The purpose for the bag is to keep the surface of the magnet free from iron particles which are very difficult to get off.
e. Move the bagged magnet around in the slurry of the cereal.
f. After a minute, take the magnet and its plastic bag out of the slurry, and examine it to see small, dark specks attached to the plastic at the magnet. This is metallic iron.
g. Unfortunately, our bodies can not absorb metallic iron very well, so this really does not help with our intake of iron. It would be better to take a supplementary multi-vitamin/mineral pill which contains an absorbable iron. The iron is needed to help form hemoglobin, which is the pigment in red blood cells responsible for transporting oxygen.
h. You can now turn the bag outside in and carefully remove the magnet from the zip-lock bag. This will keep the iron filings inside the bag and off the magnet.
Conclusions
What did you find out? Do you now have a fairly extensive list of things magnets can and cannot attract?
Subscribe to:
Comments (Atom)

.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)